- Record: found
- Abstract: found
- Article: found
Chemical Kinetics and Mass Action in Coexisting Phases

Read this article at
Abstract
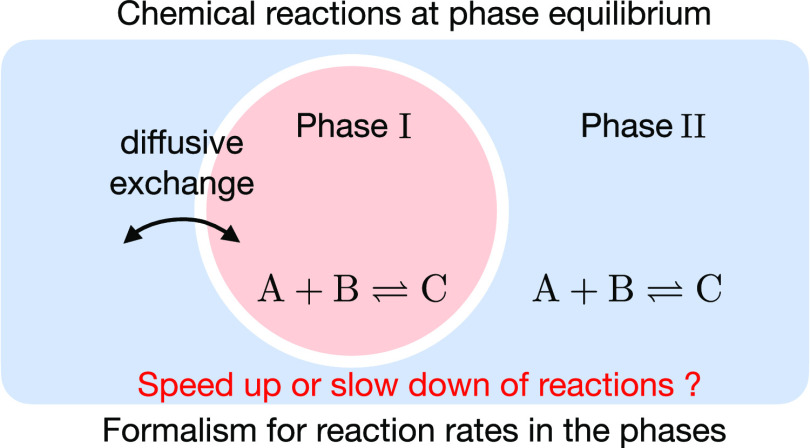
The kinetics of chemical reactions are determined by the law of mass action, which has been successfully applied to homogeneous, dilute mixtures. At nondilute conditions, interactions among the components can give rise to coexisting phases, which can significantly alter the kinetics of chemical reactions. Here, we derive a theory for chemical reactions in coexisting phases at phase equilibrium. We show that phase equilibrium couples the rates of chemical reactions of components with their diffusive exchanges between the phases. Strikingly, the chemical relaxation kinetics can be represented as a flow along the phase equilibrium line in the phase diagram. A key finding of our theory is that differences in reaction rates between coexisting phases stem solely from phase-dependent reaction rate coefficients. Our theory is key to interpreting how concentration levels of reactive components in condensed phases control chemical reaction rates in synthetic and biological systems.
Related collections
Most cited references55
- Record: found
- Abstract: found
- Article: not found
Biomolecular condensates: organizers of cellular biochemistry
- Record: found
- Abstract: found
- Article: not found
Liquid phase condensation in cell physiology and disease.
- Record: found
- Abstract: not found
- Article: not found