- Record: found
- Abstract: found
- Article: found
Characterization of developmental and molecular factors underlying release heterogeneity at Drosophila synapses
Read this article at
Abstract
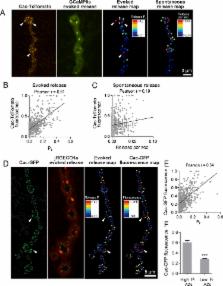
Neurons communicate through neurotransmitter release at specialized synaptic regions known as active zones (AZs). Using biosensors to visualize single synaptic vesicle fusion events at Drosophila neuromuscular junctions, we analyzed the developmental and molecular determinants of release probability ( P r ) for a defined connection with ~300 AZs. P r was heterogeneous but represented a stable feature of each AZ. P r remained stable during high frequency stimulation and retained heterogeneity in mutants lacking the Ca 2+ sensor Synaptotagmin 1. P r correlated with both presynaptic Ca 2+ channel abundance and Ca 2+ influx at individual release sites. P r heterogeneity also correlated with glutamate receptor abundance, with high P r connections developing receptor subtype segregation. Intravital imaging throughout development revealed that AZs acquire high P r during a multi-day maturation period, with P r heterogeneity largely reflecting AZ age. The rate of synapse maturation was activity-dependent, as both increases and decreases in neuronal activity modulated glutamate receptor field size and segregation.
eLife digest
To send a message to its neighbor, a neuron releases chemicals called neurotransmitters into the gap – or synapse – between them. The neurotransmitter molecules bind to proteins on the receiver neuron called receptors. But what causes the sender neuron to release neurotransmitter in the first place? The process starts when an electrical impulse called an action potential arrives at the sender cell. Its arrival causes channels in the membrane of the sender neuron to open, so that calcium ions flood into the cell. The calcium ions interact with packages of neurotransmitter molecules, known as synaptic vesicles. This causes some of the vesicles to empty their contents into the synapse.
But this process is not particularly reliable. Only a small fraction of action potentials cause vesicles to fuse with the synaptic membrane. How likely this is to occur varies greatly between neurons, and even between synapses formed by the same neuron. Synapses that are likely to release neurotransmitter are said to be strong. They are good at passing messages from the sender neuron to the receiver. Synapses with a low probability of release are said to be weak. But what exactly differs between strong and weak synapses?
Akbergenova et al. studied synapses between motor neurons and muscle cells in the fruit fly Drosophila. Each motor neuron forms several hundred synapses. Some of these synapses are 50 times more likely to release neurotransmitter than others. Using calcium imaging and genetics, Akbergenova et al. showed that sender cells at strong synapses have more calcium channels than sender cells at weak synapses. The subtypes and arrangement of receptor proteins also differ between the receiver neurons of strong versus weak synapses. Finally, studies in larvae revealed that newly formed synapses all start out weak and then gradually become stronger. How fast this strengthening occurs depends on how active the neuron at the synapse is.
This study has shown, in unprecedented detail, key molecular factors that make some fruit fly synapses more likely to release neurotransmitter than others. Many proteins at synapses of mammals resemble those at fruit fly synapses. This means that similar factors may also explain differences in synaptic strength in the mammalian brain. Changes in the strength of synapses underlie the ability to learn. Furthermore, many neurological and psychiatric disorders result from disruption of synapses. Understanding the molecular basis of synapses will thus provide clues to the origins of certain brain diseases.
Related collections
Most cited references111

- Record: found
- Abstract: found
- Article: found
Sensitive red protein calcium indicators for imaging neural activity
- Record: found
- Abstract: found
- Article: not found
Quantitative ultrastructural analysis of hippocampal excitatory synapses.
- Record: found
- Abstract: found
- Article: not found