- Record: found
- Abstract: found
- Article: found
Decreased Expression of CXCR4 Chemokine Receptor in Bone Marrow after Chemotherapy in Patients with Non-Hodgkin Lymphomas Is a Good Prognostic Factor
Read this article at
Abstract
Background
CXCR4 chemokine receptor is constitutively expressed on normal and malignant B lymphocytes derived from patients with B-cell lymphoproliferative disorders and has a significant role in cell migration to lymph nodes and bone marrow. Non-Hodgkin's lymphomas (NHL) constitute a heterogeneous group of lymphoproliferative diseases, which can localize not only to lymph nodes, but also can migrate to peripheral blood and metastase to other organs, including bone marrow.
Aim
The purpose of this study was to determine CXCR4 gene expression in peripheral blood and bone marrow of NHL patients before and after treatment.
Methods
Samples of lymphoma lymph nodes, peripheral blood and bone marrow aspirates of patients with B-cell NHL were taken at diagnosis and after chemotherapy. Gene expression was determined by the reverse transcription (RT)-polymerase chain reaction method. Expression was estimated from 0 AU (no amplificate signal) to 3 AU (maximal amplificate signal).
Results
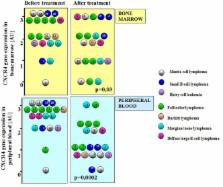
No significant difference in the level of CXCR4 expression was found in reactive lymph nodes compared to lymphoma samples We observed high level of CXCR4 expression in most patients before treatment: in bone marrow: 3 AU-10 pts, 2 AU–8 pts, 1 AU–2 pts. In peripheral blood: 3 AU–14 pts, 2 AU–4 pts, 1 AU–1 pts, 0 AU–1 pts. After chemotherapy, significant decrease in CXCR4 expression was observed. Bone marrow: 3 AU–5 pts, 2 AU–7 pts, 1 AU–5 pts, 0 AU–3 pts (p = 0.03). Peripheral blood: 3 AU–2 pts, 2 AU–6 pts, 1 AU–10 pts, 0 AU–2 pts (p = 0.0002). There was a good response to treatment in patients with significant decrease of CXCR4 expression in the bone marrow after treatment with 10-fold lower risk of death (p = 0.03).
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4.
- Record: found
- Abstract: found
- Article: not found
Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma.
- Record: found
- Abstract: found
- Article: not found