- Record: found
- Abstract: found
- Article: not found
miR‐193b represses influenza A virus infection by inhibiting Wnt/β‐catenin signalling
Read this article at
Abstract
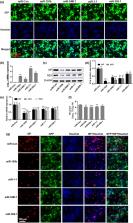
Due to an increasing emergence of new and drug‐resistant strains of the influenza A virus (IAV), developing novel measures to combat influenza is necessary. We have previously shown that inhibiting Wnt/β‐catenin pathway reduces IAV infection. In this study, we aimed to identify antiviral human microRNAs (miRNAs) that target the Wnt/β‐catenin signalling pathway. Using a miRNA expression library, we identified 85 miRNAs that up‐regulated and 20 miRNAs that down‐regulated the Wnt/β‐catenin signalling pathway. Fifteen miRNAs were validated to up‐regulate and five miRNAs to down‐regulate the pathway. Overexpression of four selected miRNAs (miR‐193b, miR‐548f‐1, miR‐1‐1, and miR‐509‐1) that down‐regulated the Wnt/β‐catenin signalling pathway reduced viral mRNA, protein levels in A/PR/8/34‐infected HEK293 cells, and progeny virus production. Overexpression of miR‐193b in lung epithelial A549 cells also resulted in decreases of A/PR/8/34 infection. Furthermore, miR‐193b inhibited the replication of various strains, including H1N1 (A/PR/8/34, A/WSN/33, A/Oklahoma/3052/09) and H3N2 (A/Oklahoma/309/2006), as determined by a viral reporter luciferase assay. Further studies revealed that β‐catenin was a target of miR‐193b, and β‐catenin rescued miR‐193b‐mediated suppression of IAV infection. miR‐193b induced G0/G1 cell cycle arrest and delayed vRNP nuclear import. Finally, adenovirus‐mediated gene transfer of miR‐193b to the lung reduced viral load in mice challenged by a sublethal dose of A/PR/8/34. Collectively, our findings suggest that miR‐193b represses IAV infection by inhibiting Wnt/β‐catenin signalling.
Related collections
Most cited references69
- Record: found
- Abstract: found
- Article: not found
Ribo-gnome: the big world of small RNAs.
- Record: found
- Abstract: found
- Article: not found
Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1.
- Record: found
- Abstract: found
- Article: not found
