- Record: found
- Abstract: found
- Article: found
Clinical remission in severe asthma with biologic therapy: an analysis from the UK Severe Asthma Registry
Read this article at
Abstract
Background
Novel biologic therapies have revolutionised the management of severe asthma with more ambitious treatment aims. Here we analyse the definition of clinical remission as a suggested treatment goal and consider the characteristics associated with clinical remission in a large, real-world severe asthma cohort.
Methods
This was a retrospective analysis of severe asthma patients registered in the UK Severe Asthma Registry (UKSAR) who met strict national access criteria for biologics. Patients had a pre-biologics baseline assessment and annual review. The primary definition of clinical remission applied included Asthma Control Questionnaire (ACQ)-5 <1.5 and no oral corticosteroids for disease control and forced expiratory volume in 1 s above lower limit of normal or no more than 100 mL less than baseline.
Results
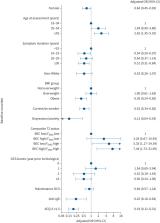
18.3% of patients achieved the primary definition of remission. The adjusted odds of remission on biologic therapy were 7.44 (95% CI 1.73–31.95)-fold higher in patients with type 2 (T2)-high biomarkers. The adjusted odds of remission were lower in patients who were female (OR 0.61, 95% CI 0.45–0.93), obese (OR 0.49, 95% CI 0.24–0.65) or had ACQ-5 ≥1.5 (OR 0.19, 95% CI 0.12–0.31) pre-biologic therapy. The likelihood of remission reduced by 14% (95% CI 0.76–0.97) for every 10-year increase in disease duration. 12–21% of the cohort attained clinical remission depending on the definition applied; most of those who did not achieve remission failed to meet multiple criteria.
Tweetable abstract
Analysis of a real-world severe asthma registry shows clinical remission rates of 18%; associated pre-biologic characteristics include male sex, never smoking, BMI <30 kg·m −2, shorter disease duration, T2-high biomarkers and lower symptom burden https://bit.ly/46JLeDb
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations.
- Record: found
- Abstract: found
- Article: not found
International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma.
- Record: found
- Abstract: found
- Article: not found