- Record: found
- Abstract: found
- Article: found
Long Noncoding RNA CRNDE/PRC2 Participated in the Radiotherapy Resistance of Human Lung Adenocarcinoma Through Targeting p21 Expression
Read this article at
Abstract
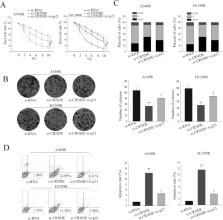
Long noncoding RNAs (lncRNAs), a new class of functional regulators involved in human tumorigenesis, have been attracting the increasing attention of researchers. The lncRNA colorectal neoplasia differentially expressed (CRNDE) gene, transcribed from chromosome 16 on the strand opposite the adjacent IRX5 gene, was originally found to be increased in CRC and was reported to be abnormally expressed in many cancers. However, its potential role and the molecular mechanism underlying the radioresistant phenotype formation of lung adenocarcinoma (LAD) remain unclear. In our present study, we identified that CRNDE was significantly upregulated in LAD tissue and radioresistant LAD cell lines. A high level of CRNDE expression was significantly correlated with poor differentiation, TNM stage, lymph node metastasis, radiotherapy response, and a significantly shorter overall survival. Gain- and loss-of-function tests revealed that CRNDE could influence the radiosensitivity of LAD cells by affecting the G 1/S transition and causing apoptosis of LAD cells in vitro. Additionally, the mechanistic investigations showed that CRNDE could interact with PRC2 and recruit its core component EZH2 to p21 (CDKN1A) promoter regions and repress its transcription. Furthermore, rescue experiments were performed to confirm that CRNDE oncogenic function was partly through regulating p21. In conclusion, our data suggest that CRNDE may function as an oncogene by modulating p21, finally contributing to the radioresistant phenotype formation of LAD cells.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Long non-coding RNAs: insights into functions.
- Record: found
- Abstract: found
- Article: not found
Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression.
- Record: found
- Abstract: found
- Article: not found