- Record: found
- Abstract: found
- Article: found
Viral load dynamics of SARS-CoV-2 Delta and Omicron variants following multiple vaccine doses and previous infection
Read this article at
Abstract
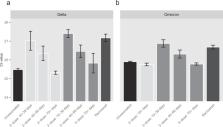
An important aspect of vaccine effectiveness is its impact on pathogen transmissibility, harboring major implications for public health policies. As viral load is a prominent factor affecting infectivity, its laboratory surrogate, qRT-PCR cycle threshold (Ct), can be used to investigate the infectivity-related component of vaccine effectiveness. While vaccine waning has previously been observed for viral load during the Delta wave, less is known regarding how Omicron viral load is affected by vaccination status, and whether vaccine-derived and natural infection protection are sustained. By analyzing results of more than 460,000 individuals, we show that while recent vaccination reduces Omicron viral load, its effect wanes rapidly. In contrast, a significantly slower waning rate is demonstrated for recovered COVID-19 individuals. Thus, while the vaccine is effective in decreasing morbidity and mortality, its relatively small effect on transmissibility of Omicron (as measured here by Ct) and its rapid waning call for reassessment of future booster campaigns.
Abstract
COVID vaccination can reduce virus levels in breakthrough infections, which in turn may reduce transmission of the virus. By using qRT-PCR cycle threshold as a surrogate of virus levels, the authors here show that this positive effect of vaccination wanes relatively quickly for Omicron breakthrough infection.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting
- Record: found
- Abstract: found
- Article: not found
Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months
- Record: found
- Abstract: found
- Article: not found