- Record: found
- Abstract: found
- Article: found
Durable natural killer cell response after three doses of SARS-CoV-2 inactivated vaccine in HIV-infected individuals
Read this article at
Abstract
Background:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine can induce a potent cellular and humoral immune response to protect against SARS-CoV-2 infection. However, it was unknown whether SARS-CoV-2 vaccination can induce effective natural killer (NK) cell response in people living with human immunodeficiency virus (PLWH) and healthy individuals.
Methods:
Forty-seven PLWH and thirty healthy controls (HCs) inoculated with SARS-CoV-2 inactivated vaccine were enrolled from Beijing Youan Hospital in this study. The effect of SARS-CoV-2 vaccine on NK cell frequency, phenotype, and function in PLWH and HCs was evaluated by flow cytometry, and the response of NK cells to SARS-CoV-2 Omicron Spike (SARS-2-OS) protein stimulation was also evaluated.
Results:
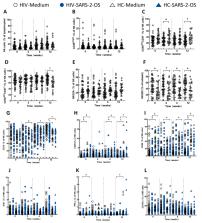
SARS-CoV-2 vaccine inoculation elicited activation and degranulation of NK cells in PLWH, which peaked at 2 weeks and then decreased to a minimum at 12 weeks after the third dose of vaccine. However, in vitro stimulation of the corresponding peripheral blood monocular cells from PLWH with SARS-2-OS protein did not upregulate the expression of the aforementioned markers. Additionally, the frequencies of NK cells expressing the activation markers CD25 and CD69 in PLWH were significantly lower than those in HCs at 0, 4 and 12 weeks, but the percentage of CD16 + NK cells in PLWH was significantly higher than that in HCs at 2, 4 and 12 weeks after the third dose of vaccine. Interestingly, the frequency of CD16 + NK cells was significantly negatively correlated with the proportion of CD107a + NK cells in PLWH at each time point after the third dose. Similarly, this phenomenon was also observed in HCs at 0, 2, and 4 weeks after the third dose. Finally, regardless of whether NK cells were stimulated with SARS-2-OS or not, we did not observe any differences in the expression of NK cell degranulation markers between PLWH and HCs.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Exploring the NK cell platform for cancer immunotherapy
- Record: found
- Abstract: found
- Article: not found
NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17).
- Record: found
- Abstract: found
- Article: not found