- Record: found
- Abstract: found
- Article: found
Epigenetic Characterization of the FMR1 Gene and Aberrant Neurodevelopment in Human Induced Pluripotent Stem Cell Models of Fragile X Syndrome
Read this article at
Abstract
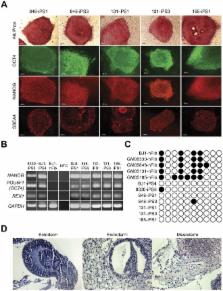
Fragile X syndrome (FXS) is the most common inherited cause of intellectual disability. In addition to cognitive deficits, FXS patients exhibit hyperactivity, attention deficits, social difficulties, anxiety, and other autistic-like behaviors. FXS is caused by an expanded CGG trinucleotide repeat in the 5′ untranslated region of the Fragile X Mental Retardation ( FMR1) gene leading to epigenetic silencing and loss of expression of the Fragile X Mental Retardation protein ( FMRP). Despite the known relationship between FMR1 CGG repeat expansion and FMR1 silencing, the epigenetic modifications observed at the FMR1 locus, and the consequences of the loss of FMRP on human neurodevelopment and neuronal function remain poorly understood. To address these limitations, we report on the generation of induced pluripotent stem cell (iPSC) lines from multiple patients with FXS and the characterization of their differentiation into post-mitotic neurons and glia. We show that clones from reprogrammed FXS patient fibroblast lines exhibit variation with respect to the predominant CGG-repeat length in the FMR1 gene. In two cases, iPSC clones contained predominant CGG-repeat lengths shorter than measured in corresponding input population of fibroblasts. In another instance, reprogramming a mosaic patient having both normal and pre-mutation length CGG repeats resulted in genetically matched iPSC clonal lines differing in FMR1 promoter CpG methylation and FMRP expression. Using this panel of patient-specific, FXS iPSC models, we demonstrate aberrant neuronal differentiation from FXS iPSCs that is directly correlated with epigenetic modification of the FMR1 gene and a loss of FMRP expression. Overall, these findings provide evidence for a key role for FMRP early in human neurodevelopment prior to synaptogenesis and have implications for modeling of FXS using iPSC technology. By revealing disease-associated cellular phenotypes in human neurons, these iPSC models will aid in the discovery of novel therapeutics for FXS and other autism-spectrum disorders sharing common pathophysiology.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Reprogramming of human somatic cells to pluripotency with defined factors.
- Record: found
- Abstract: found
- Article: not found
Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome.
- Record: found
- Abstract: found
- Article: not found