- Record: found
- Abstract: found
- Article: not found
Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs)
Read this article at
Abstract
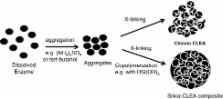
Cross-linked enzyme aggregates (CLEAs) have many economic and environmental benefits in the context of industrial biocatalysis. They are easily prepared from crude enzyme extracts, and the costs of (often expensive) carriers are circumvented. They generally exhibit improved storage and operational stability towards denaturation by heat, organic solvents, and autoproteolysis and are stable towards leaching in aqueous media. Furthermore, they have high catalyst productivities (kilograms product per kilogram biocatalyst) and are easy to recover and recycle. Yet another advantage derives from the possibility to co-immobilize two or more enzymes to provide CLEAs that are capable of catalyzing multiple biotransformations, independently or in sequence as catalytic cascade processes.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking.
- Record: found
- Abstract: found
- Article: not found
Glucose oxidase--an overview.
- Record: found
- Abstract: found
- Article: not found
