- Record: found
- Abstract: found
- Article: not found
Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1
Read this article at
Abstract
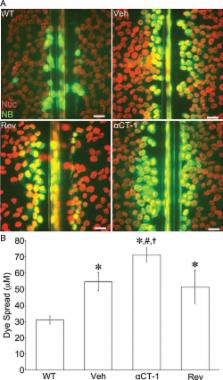
Cx43 gap junctions (GJs) are integral to the function of the mammalian heart. It is shown that ZO-1 dynamically regulates the transition between Cx43 connexons and GJ intercellular channels, determining the balance of connexon-mediated cell permeability to GJ communication. Importantly, a novel domain proximal to GJs is identified—the perinexus.
Abstract
Connexin 43 (Cx43) is a gap junction (GJ) protein widely expressed in mammalian tissues that mediates cell-to-cell coupling. Intercellular channels comprising GJ aggregates form from docking of paired connexons, with one each contributed by apposing cells. Zonula occludens-1 (ZO-1) binds the carboxy terminus of Cx43, and we have previously shown that inhibition of the Cx43/ZO-1 interaction increases GJ size by 48 h. Here we demonstrated that increases in GJ aggregation occur within 2 h (∼Cx43 half-life) following disruption of Cx43/ZO-1. Immunoprecipitation and Duolink protein–protein interaction assays indicated that inhibition targets ZO-1 binding with Cx43 in GJs as well as connexons in an adjacent domain that we term the “perinexus.” Consistent with GJ size increases being matched by decreases in connexons, inhibition of Cx43/ZO-1 reduced the extent of perinexal interaction, increased the proportion of connexons docked in GJs relative to undocked connexons in the plasma membrane, and increased GJ intercellular communication while concomitantly decreasing hemichannel-mediated membrane permeance in contacting, but not noncontacting, cells. ZO-1 small interfering RNA and overexpression experiments verified that loss and gain of ZO-1 function govern the transition of connexons into GJs. It is concluded that ZO-1 regulates the rate of undocked connexon aggregation into GJs, enabling dynamic partitioning of Cx43 channel function between junctional and proximal nonjunctional domains of plasma membrane.
Related collections
Most cited references61

- Record: found
- Abstract: found
- Article: found
Remodelling of gap junctions and connexin expression in diseased myocardium
- Record: found
- Abstract: found
- Article: not found
The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking.
- Record: found
- Abstract: found
- Article: not found
