- Record: found
- Abstract: found
- Article: not found
Connexins in Cancer: Bridging the Gap to the Clinic
Read this article at
Abstract
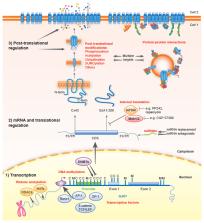
Gap junctions comprise arrays of intercellular channels formed by connexin proteins and provide for direct communication between adjacent cells. This type of intercellular communication permits coordination of cellular activity and plays key roles in the control of cell growth and differentiation and in the maintenance of tissue homeostasis. After more than 50 years deciphering the links among connexins, gap junctions and cancer, researchers are now beginning to translate this knowledge to the clinic. The emergence of new strategies for connexin targeting, combined with improved understanding of the molecular bases underlying the dysregulation of connexins during cancer development, offer novel opportunities for clinical applications. However, different connexin isoforms have diverse channel-dependent and -independent functions that are tissue- and stage-specific. This can elicit both pro- and anti-tumorigenic effects that engender significant challenges in the path towards personalised medicine. Here, we review the current understanding of the role of connexins and gap junctions in cancer, with a particular focus on recent progress made in determining their prognostic and therapeutic potential.
Related collections
Most cited references212
- Record: found
- Abstract: found
- Article: not found
Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer
- Record: found
- Abstract: found
- Article: not found
Brain tumour cells interconnect to a functional and resistant network.
- Record: found
- Abstract: found
- Article: found
