- Record: found
- Abstract: found
- Article: found
Cross-Serotype Immunity Induced by Immunization with a Conserved Rhinovirus Capsid Protein
Read this article at
Abstract
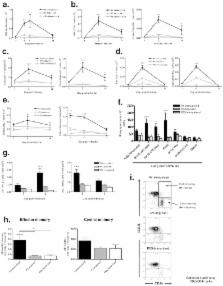
Human rhinovirus (RV) infections are the principle cause of common colds and precipitate asthma and COPD exacerbations. There is currently no RV vaccine, largely due to the existence of ∼150 strains. We aimed to define highly conserved areas of the RV proteome and test their usefulness as candidate antigens for a broadly cross-reactive vaccine, using a mouse infection model. Regions of the VP0 (VP4+VP2) capsid protein were identified as having high homology across RVs. Immunization with a recombinant VP0 combined with a Th1 promoting adjuvant induced systemic, antigen specific, cross-serotype, cellular and humoral immune responses. Similar cross-reactive responses were observed in the lungs of immunized mice after infection with heterologous RV strains. Immunization enhanced the generation of heterosubtypic neutralizing antibodies and lung memory T cells, and caused more rapid virus clearance. Conserved domains of the RV capsid therefore induce cross-reactive immune responses and represent candidates for a subunit RV vaccine.
Author Summary
Human rhinovirus infections cause the majority of common colds as well as asthma and chronic obstructive pulmonary disease (COPD) exacerbations. The disease burden attributable to rhinoviruses is therefore huge. Despite this and the fact that human rhinoviruses were discovered over 50 years ago, there are currently no specific antiviral therapies or vaccine available. The lack of a rhinovirus vaccine can at least in part be attributed to the fact that rhinoviruses like other pathogens have high variability in surface antibody binding regions, resulting in >100 serotypically distinct strains. We have defined areas of the rhinovirus polyprotein which are highly conserved across strains and which may therefore induce cross-reactive immune responses capable of providing broader protection. Using a mouse model, we show that immunization with a recombinant rhinovirus capsid protein induces cross-reactive cellular and humoral immune responses. After subsequent infection, immunization enhances both neutralising antibody and lung effector and memory T cell responses, expediting virus clearance. Importantly these effects were evident upon challenge with multiple heterologous rhinovirus serotypes, indicating that immunization with conserved rhinovirus capsid proteins may represent a viable strategy for producing a broadly cross-reactive vaccine.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Community study of role of viral infections in exacerbations of asthma in 9-11 year old children.
- Record: found
- Abstract: found
- Article: not found
SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny.
- Record: found
- Abstract: found
- Article: not found