- Record: found
- Abstract: found
- Article: found
Small soluble α-synuclein aggregates are the toxic species in Parkinson’s disease
Read this article at
Abstract
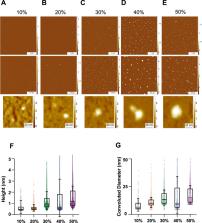
Soluble α-synuclein aggregates varying in size, structure, and morphology have been closely linked to neuronal death in Parkinson’s disease. However, the heterogeneity of different co-existing aggregate species makes it hard to isolate and study their individual toxic properties. Here, we show a reliable non-perturbative method to separate a heterogeneous mixture of protein aggregates by size. We find that aggregates of wild-type α-synuclein smaller than 200 nm in length, formed during an in vitro aggregation reaction, cause inflammation and permeabilization of single-liposome membranes and that larger aggregates are less toxic. Studying soluble aggregates extracted from post-mortem human brains also reveals that these aggregates are similar in size and structure to the smaller aggregates formed in aggregation reactions in the test tube. Furthermore, we find that the soluble aggregates present in Parkinson’s disease brains are smaller, largely less than 100 nm, and more inflammatory compared to the larger aggregates present in control brains. This study suggests that the small non-fibrillar α-synuclein aggregates are the critical species driving neuroinflammation and disease progression.
Abstract
α-synuclein aggregates cause neuronal damage, but their heterogeneity complicates studying their toxic properties. Here, the authors analyze α-synuclein aggregates in vitro and study post-mortem brain samples, providing evidence that small aggregates are the main culprit for neuronal death in Parkinson’s disease.
Related collections
Most cited references68
- Record: found
- Abstract: found
- Article: not found
Parkinson disease
- Record: found
- Abstract: not found
- Article: not found
Staging of brain pathology related to sporadic Parkinson’s disease
- Record: found
- Abstract: found
- Article: not found