- Record: found
- Abstract: found
- Article: found
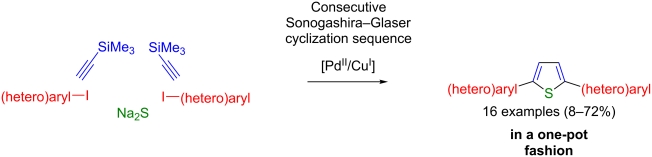
Pseudo five-component synthesis of 2,5-di(hetero)arylthiophenes via a one-pot Sonogashira–Glaser cyclization sequence
research-article

Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Summary
Based upon a consecutive one-pot Sonogashira–Glaser coupling–cyclization sequence a variety of 2,5-di(hetero)arylthiophenes were synthesized in moderate to good yields. A single Pd/Cu-catalyst system, without further catalyst addition, and easily available, stable starting materials were used, resulting in a concise and highly efficient route for the synthesis of the title compounds. This novel pseudo five-component synthesis starting from iodo(hetero)arenes is particularly suitable as a direct access to well-defined thiophene oligomers, which are of peculiar interest in materials science.
Abstract
Related collections
Most cited references26
- Record: found
- Abstract: not found
- Article: not found
Functional oligothiophenes: molecular design for multidimensional nanoarchitectures and their applications.
Amaresh Mishra, Chang-Qi Ma, Peter Bäuerle (2009)
- Record: found
- Abstract: not found
- Article: not found
Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst
Norio Miyaura, Akira Suzuki (1979)