- Record: found
- Abstract: found
- Article: found
Copper single-atom catalysts with photothermal performance and enhanced nanozyme activity for bacteria‐infected wound therapy
Read this article at
Abstract
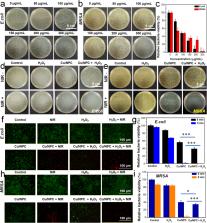
Nanozymes have become a new generation of antibiotics with exciting broad-spectrum antibacterial properties and negligible biological toxicity. However, their inherent low catalytic activity limits their antibacterial properties. Herein, Cu single-atom sites/N doped porous carbon (Cu SASs/NPC) is successfully constructed for photothermal-catalytic antibacterial treatment by a pyrolysis-etching-adsorption-pyrolysis (PEAP) strategy. Cu SASs/NPC have stronger peroxidase-like catalytic activity, glutathione (GSH)-depleting function, and photothermal property compared with non-Cu-doped NPC, indicating that Cu doping significantly improves the catalytic performance of nanozymes. Cu SASs/NPC can effectively induce peroxidase-like activity in the presence of H 2O 2, thereby generating a large amount of hydroxyl radicals (•OH), which have a certain killing effect on bacteria and make bacteria more susceptible to temperature. The introduction of near-infrared (NIR) light can generate hyperthermia to fight bacteria, and enhance the peroxidase-like catalytic activity, thereby generating additional •OH to destroy bacteria. Interestingly, Cu SASs/NPC can act as GSH peroxidase (GSH-Px)-like nanozymes, which can deplete GSH in bacteria, thereby significantly improving the sterilization effect. PTT-catalytic synergistic antibacterial strategy produces almost 100% antibacterial efficiency against Escherichia coli ( E. coli) and methicillin-resistant Staphylococcus aureus ( MRSA). In vivo experiments show a better PTT-catalytic synergistic therapeutic performance on MRSA-infected mouse wounds. Overall, our work highlights the wide antibacterial and anti-infective bio-applications of Cu single-atom-containing catalysts.
Graphical abstract
Highlights
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Targeting microbial biofilms: current and prospective therapeutic strategies.
- Record: found
- Abstract: found
- Article: not found
Self-Assembled Copper-Amino Acid Nanoparticles for In Situ Glutathione “AND” H2O2 Sequentially Triggered Chemodynamic Therapy
- Record: found
- Abstract: found
- Article: not found
