- Record: found
- Abstract: found
- Article: found
CAFs orchestrates tumor immune microenvironment—A new target in cancer therapy?
Read this article at
Abstract
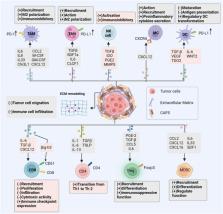
Cancer immunotherapy has opened a new landscape in cancer treatment, however, the poor specificity and resistance of most targeted therapeutics have limited their therapeutic efficacy. In recent years, the role of CAFs in immune regulation has been increasingly noted as more evidence has been uncovered regarding the link between cancer-associated fibroblasts (CAFs) and the evolutionary process of tumor progression. CAFs interact with immune cells to shape the tumor immune microenvironment (TIME) that favors malignant tumor progression, a crosstalk process that leads to the failure of cancer immunotherapies. In this review, we outline recent advances in the immunosuppressive function of CAFs, highlight the mechanisms of CAFs-immune cell interactions, and discuss current CAF-targeted therapeutic strategies for future study.
Related collections
Most cited references191
- Record: found
- Abstract: found
- Article: not found
TGF-β attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells
- Record: found
- Abstract: found
- Article: not found