- Record: found
- Abstract: found
- Article: found
Impact of mature tertiary lymphoid structures on prognosis and therapeutic response of Epstein-Barr virus-associated gastric cancer patients
Read this article at
Abstract
Background
Epstein-Barr virus-associated gastric cancer (EBVaGC) exhibits unique histological characteristics within the immune-cell-rich microenvironment, but the role of tertiary lymphoid structure (TLS) in EBVaGC is not yet fully understood.
Methods
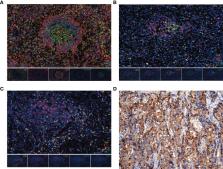
We retrospectively identified EBVaGC from 8517 consecutive GC cases from the two top cancer centers in China. Furthermore, we evaluated the prognostic value of TLS in 148 EBVaGC patients from our institute and then validated it in an external cohort (76 patients). TLS was quantified and its relationships with overall survival (OS) and therapeutic response were further analyzed. Multiplex immunofluorescence staining and targeted sequencing were used to characterize the composition of TLS and the genomic landscape, respectively.
Results
In our study, EBVaGC was observed in 4.3% (190/4436) and 2.6% (109/4081) of GCs in the training and validation cohorts, respectively. TLS was identified in the intratumor (94.6%) and peritumor (77.0%) tissues with lymphoid aggregates, primary and secondary (i.e., mature TLSs) follicles in EBVaGC. Kaplan-Meier analysis showed that mature TLS in intratumoral tissues was associated with a favorable OS in the training and validation cohorts (p < 0.0001; p = 0.0108). Multivariate analyses demonstrated that intratumoral TLS maturation, pTNM, and PD-L1 expression were independent prognostic factors for OS (p < 0.05). Furthermore, the mature TLS was significantly associated with a good response to treatment in EBVaGC patients. Interestingly, the mutation frequency of SMARCA4 was significantly lower in the mature TLS groups.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer.
- Record: found
- Abstract: found
- Article: not found
Gastric cancer

- Record: found
- Abstract: found
- Article: found