- Record: found
- Abstract: found
- Article: found
HCMV IE1/IE1mut Therapeutic Vaccine Induces Tumor Regression via Intratumoral Tertiary Lymphoid Structure Formation and Peripheral Immunity Activation in Glioblastoma Multiforme
Read this article at
Abstract
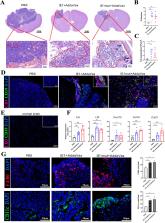
Glioblastoma multiforme (GBM), a highly malignant invasive brain tumor, is associated with poor prognosis and survival and lacks an effective cure. High expression of the human cytomegalovirus (HCMV) immediate early protein 1 (IE1) in GBM tissues is strongly associated with their malignant progression, presenting a novel target for therapeutic strategies. Here, the bioluminescence imaging technology revealed remarkable tumor shrinkage and improved survival rates in a mouse glioma model treated with HCMV IE1/IE1mut vaccine. In addition, immunofluorescence data demonstrated that the treated group exhibited significantly more and larger tertiary lymphoid structures (TLSs) than the untreated group. The presence of TLS was associated with enhanced T cell infiltration, and a large number of proliferating T cells were found in the treated group. Furthermore, the flow cytometry results showed that in the treatment group, cytotoxic T lymphocytes exhibited partial polarization toward effector memory T cells and were activated to play a lethal role in the peripheral immunological organs. Furthermore, a substantial proportion of B cells in the draining lymph nodes expressed CD40 and CD86. Surprisingly, quantitative polymerase chain reaction indicated that a high expression of cytokines, including chemokines in brain tumors and immune tissues, induced the differentiation, development, and chemokine migration of immune cells in the treated group. Our study data demonstrate that IE1 or IE1mut vaccination has a favorable effect in glioma mice models. This study holds substantial implications for identifying new and effective therapeutic targets within GBM.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
T cell exclusion, immune privilege, and the tumor microenvironment.
- Record: found
- Abstract: found
- Article: not found
Tertiary lymphoid structures in the era of cancer immunotherapy
- Record: found
- Abstract: not found
- Article: not found