- Record: found
- Abstract: found
- Article: found
Tertiary lymphoid structures in cancer: maturation and induction
Read this article at
Abstract
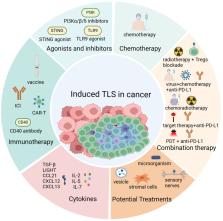
Tertiary lymphoid structure (TLS) is an ectopic lymphocyte aggregate formed in peripheral non-lymphoid tissues, including inflamed or cancerous tissue. Tumor-associated TLS serves as a prominent center of antigen presentation and adaptive immune activation within the periphery, which has exhibited positive prognostic value in various cancers. In recent years, the concept of maturity regarding TLS has been proposed and mature TLS, characterized by well-developed germinal centers, exhibits a more potent tumor-suppressive capacity with stronger significance. Meanwhile, more and more evidence showed that TLS can be induced by therapeutic interventions during cancer treatments. Thus, the evaluation of TLS maturity and the therapeutic interventions that induce its formation are critical issues in current TLS research. In this review, we aim to provide a comprehensive summary of the existing classifications for TLS maturity and therapeutic strategies capable of inducing its formation in tumors.
Related collections
Most cited references115

- Record: found
- Abstract: found
- Article: found
Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression
- Record: found
- Abstract: found
- Article: not found
B cells and tertiary lymphoid structures promote immunotherapy response
- Record: found
- Abstract: found
- Article: not found