- Record: found
- Abstract: found
- Article: found
Current perspectives on the health risks associated with the consumption of advanced glycation end products: recommendations for dietary management
Read this article at
Abstract
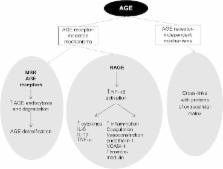
Advanced glycation end products (AGEs) constitute a complex group of compounds produced endogenously during the aging process and under conditions of hyperglycemia and oxidative stress. AGEs also have an emerging exogenous origin. Cigarette smoke and diet are the two main exogenous sources of AGEs (glycotoxins). Modern Western diets are rich in AGEs which have been implicated in the pathogenesis of several metabolic and degenerative disorders. Accumulating evidence underlies the beneficial effect of the dietary restriction of AGEs not only in animal studies but also in patients with diabetic complications and metabolic diseases. This article reviews the evidence linking dietary glycotoxins to several disorders from diabetic complications and renal failure to liver dysfunction, female reproduction, eye and cognitive disorders as well as cancer. Furthermore, strategies for AGE reduction are discussed with a focus on dietary modification.
Related collections
Most cited references89
- Record: found
- Abstract: found
- Article: not found
Clinical review: The role of advanced glycation end products in progression and complications of diabetes.
- Record: found
- Abstract: found
- Article: not found
Tobacco smoke is a source of toxic reactive glycation products.
- Record: found
- Abstract: found
- Article: not found