- Record: found
- Abstract: found
- Article: found
Common Protective Strategies in Neurodegenerative Disease: Focusing on Risk Factors to Target the Cellular Redox System
Read this article at
Abstract
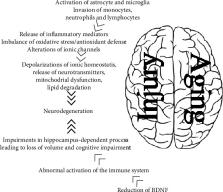
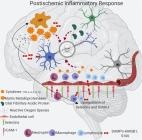
Neurodegenerative disease is an umbrella term for different conditions which primarily affect the neurons in the human brain. In the last century, significant research has been focused on mechanisms and risk factors relevant to the multifaceted etiopathogenesis of neurodegenerative diseases. Currently, neurodegenerative diseases are incurable, and the treatments available only control the symptoms or delay the progression of the disease. This review is aimed at characterizing the complex network of molecular mechanisms underpinning acute and chronic neurodegeneration, focusing on the disturbance in redox homeostasis, as a common mechanism behind five pivotal risk factors: aging, oxidative stress, inflammation, glycation, and vascular injury. Considering the complex multifactorial nature of neurodegenerative diseases, a preventive strategy able to simultaneously target multiple risk factors and disease mechanisms at an early stage is most likely to be effective to slow/halt the progression of neurodegenerative diseases.
Related collections
Most cited references182
- Record: found
- Abstract: found
- Article: not found
HMG-1 as a late mediator of endotoxin lethality in mice.
- Record: found
- Abstract: found
- Article: not found
Control of microglial neurotoxicity by the fractalkine receptor.
- Record: found
- Abstract: found
- Article: found