- Record: found
- Abstract: found
- Article: found
Superresolution microscopy localizes endogenous Dvl2 to Wnt signaling-responsive biomolecular condensates
Read this article at
Significance
Wnt signaling governs cell fate and tissue polarity across species. The Dishevelled proteins are central to Wnt signaling cascades. Wnt-mediated multiprotein complexes such as the “signalosome” and the “destruction complex” have been proposed to represent biomolecular condensates. These nonmembranous, specialized compartments have been suggested to form through liquid–liquid phase separation and ensure correctly proceeding physiological reactions. Although biomolecular condensates have increasingly been studied, key questions remain regarding, for example, their architecture and physiological regulation. Here, superresolution microscopy after endogenous labeling of Dishevelled-2 gives insights into protein functions and Wnt signaling at physiological levels. It reveals the distinct molecular architecture of endogenous Wnt condensates at single-molecule resolution and illustrates close interactions at the centrosome.
Abstract
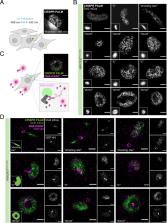
During organismal development, homeostasis, and disease, Dishevelled (Dvl) proteins act as key signaling factors in beta-catenin–dependent and beta-catenin–independent Wnt pathways. While their importance for signal transmission has been genetically demonstrated in many organisms, our mechanistic understanding is still limited. Previous studies using overexpressed proteins showed Dvl localization to large, punctate-like cytoplasmic structures that are dependent on its DIX domain. To study Dvl’s role in Wnt signaling, we genome engineered an endogenously expressed Dvl2 protein tagged with an mEos3.2 fluorescent protein for superresolution imaging. First, we demonstrate the functionality and specificity of the fusion protein in beta-catenin–dependent and beta-catenin–independent signaling using multiple independent assays. We performed live-cell imaging of Dvl2 to analyze the dynamic formation of the supramolecular cytoplasmic Dvl2_mEos3.2 condensates. While overexpression of Dvl2_mEos3.2 mimics the previously reported formation of abundant large “puncta,” supramolecular condensate formation at physiological protein levels is only observed in a subset of cells with approximately one per cell. We show that, in these condensates, Dvl2 colocalizes with Wnt pathway components at gamma-tubulin and CEP164-positive centrosomal structures and that the localization of Dvl2 to these condensates is Wnt dependent. Single-molecule localization microscopy using photoactivated localization microscopy (PALM) of mEos3.2 in combination with DNA-PAINT demonstrates the organization and repetitive patterns of these condensates in a cell cycle–dependent manner. Our results indicate that the localization of Dvl2 in supramolecular condensates is coordinated dynamically and dependent on cell state and Wnt signaling levels. Our study highlights the formation of endogenous and physiologically regulated biomolecular condensates in the Wnt pathways at single-molecule resolution.
Related collections
Most cited references107
- Record: found
- Abstract: found
- Article: not found
Biomolecular condensates: organizers of cellular biochemistry
- Record: found
- Abstract: found
- Article: not found
Wnt/β-catenin signaling and disease.
- Record: found
- Abstract: found
- Article: not found