- Record: found
- Abstract: found
- Article: found
A New Phase of Networking: The Molecular Composition and Regulatory Dynamics of Mammalian Stress Granules
Read this article at
Abstract
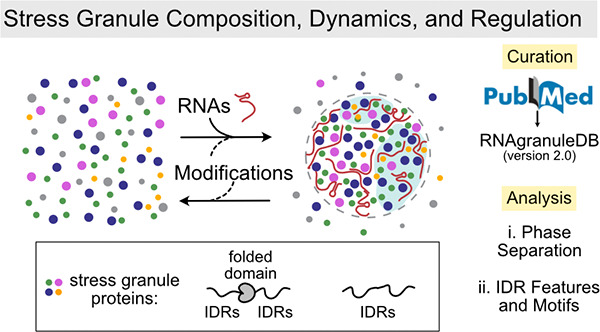
Stress granules (SGs) are cytosolic biomolecular condensates that form in response to cellular stress. Weak, multivalent interactions between their protein and RNA constituents drive their rapid, dynamic assembly through phase separation coupled to percolation. Though a consensus model of SG function has yet to be determined, their perceived implication in cytoprotective processes ( e.g. , antiviral responses and inhibition of apoptosis) and possible role in the pathogenesis of various neurodegenerative diseases ( e.g. , amyotrophic lateral sclerosis and frontotemporal dementia) have drawn great interest. Consequently, new studies using numerous cell biological, genetic, and proteomic methods have been performed to unravel the mechanisms underlying SG formation, organization, and function and, with them, a more clearly defined SG proteome. Here, we provide a consensus SG proteome through literature curation and an update of the user-friendly database RNAgranuleDB to version 2.0 ( http://rnagranuledb.lunenfeld.ca/). With this updated SG proteome, we use next-generation phase separation prediction tools to assess the predisposition of SG proteins for phase separation and aggregation. Next, we analyze the primary sequence features of intrinsically disordered regions (IDRs) within SG-resident proteins. Finally, we review the protein- and RNA-level determinants, including post-translational modifications (PTMs), that regulate SG composition and assembly/disassembly dynamics.
Related collections
Most cited references255
- Record: found
- Abstract: found
- Article: not found
Biomolecular condensates: organizers of cellular biochemistry
- Record: found
- Abstract: found
- Article: not found