- Record: found
- Abstract: found
- Article: found
Emerging insights on the role of gasdermins in infection and inflammatory diseases
Read this article at
Abstract
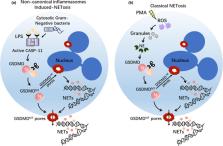
The gasdermins, family of pore‐forming proteins, are emerging key regulators of infection, autoinflammation and antitumor immunity. Multiple studies have recently characterised their crucial roles in driving pyroptosis, a lytic pro‐inflammatory type of cell death. Additionally, gasdermins also act as key effectors of NETosis, secondary necrosis and apoptosis. In this review, we will address current understanding of the mechanisms of gasdermin activation and further describe the protective and detrimental roles of gasdermins in host defence and autoinflammatory diseases. These data suggest that gasdermins play a prominent role in innate immunity and autoinflammatory disorders, thereby providing potential new therapeutic avenues for the treatment of infection and autoimmune disease.
Abstract
This review summarises the latest advances in several aspects of mode of activation of gasdermins, which finally drives pyroptosis, NETosis, secondary necrotic death and apoptosis. Here, we also highlight the current understanding of gasdermin involvement in infections and autoimmunity disorders, providing a promising translational approach to the treatment of infectious and autoinflammatory diseases.
Related collections
Most cited references87
- Record: found
- Abstract: not found
- Article: not found
Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a Gasdermin
- Record: found
- Abstract: found
- Article: not found
Gasdermin E suppresses tumor growth by activating anti-tumor immunity
- Record: found
- Abstract: found
- Article: not found
