- Record: found
- Abstract: found
- Article: found
Current hepatitis E virus seroprevalence in Swiss blood donors and apparent decline from 1997 to 2016
Read this article at
Background and aim
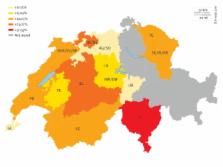
Hepatitis E virus (HEV) is a virus of emerging importance to transfusion medicine. Studies from several European countries, including Switzerland, have reported high seroprevalence of hepatitis E as a consequence of endemic infections. Published HEV seroprevalence estimates within developed countries vary considerably; primarily due to improved diagnostic assays. The purpose of this study was to investigate the seroprevalence of anti-HEV IgG in Swiss blood donations. Methods: We used the highly sensitive Wantai HEV IgG EIA and assessed regional distribution patterns. We analysed age- and sex-matched archive plasma dating back 20 years from canton Bern to investigate recent changes in HEV seroprevalence levels. Results: On average, 20.4% (95% confidence intervals: 19.1–21.8) of the 3,609 blood samples collected in 2014–16 were anti-HEV IgG positive; however, distinct differences between geographical regions were observed (range: 12.8–33.6%). Seroprevalence increased with age with 30.7% of males and 34.3% of women being positive donors over > 60 years old. Differences between sexes may be attributed to dissimilarities in the average age of this group. Within the specified region of the Bern canton, overall prevalence has declined over two decades from 30.3% in 1997/98 to 27.0% in 2006 and 22.3% in 2015/6. Conclusions: HEV seroprevalence in Switzerland is high, but has declined over the last decades. The result shows that primarily endemic HEV infections occur and that current blood products may pose a risk to vulnerable transfusion recipients. Nucleic acid screening of all blood products for HEV will begin in November 2018.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries.

- Record: found
- Abstract: found
- Article: not found