- Record: found
- Abstract: found
- Article: found
Predictive value of in vitro assays depends on the mechanism of toxicity of metal oxide nanoparticles
Read this article at
Abstract
Background
Hazard identification for risk assessment of nanoparticles (NPs) is mainly composed of in vitro cell-based assays and in vivo animal experimentation. The rapidly increasing number and functionalizations of NPs makes in vivo toxicity tests undesirable on both ethical and financial grounds, creating an urgent need for development of in vitro cell-based assays that accurately predict in vivo toxicity and facilitate safe nanotechnology.
Methods
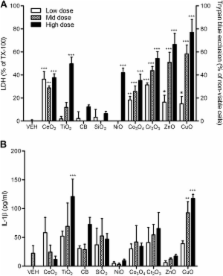
In this study, we used 9 different NPs (CeO 2, TiO 2, carbon black, SiO 2, NiO, Co 3O 4, Cr 2O 3, CuO, and ZnO). As an in vivo toxicity endpoint, the acute lung inflammogenicity in a rat instillation model was compared with the in vitro toxicity endpoints comprising cytotoxicity, pro-inflammatory cytokine expression, or haemolytic potential. For in vitro assays, 8 different cell-based assays were used including epithelial cells, monocytic/macrophage cells, human erythrocytes, and combined culture.
Results
ZnO and CuO NPs acting via soluble toxic ions showed positive results in most of assays and were consistent with the lung inflammation data. When compared in in vitro assays at the same surface area dose (30 cm 2/mL), NPs that were low solubility and therefore acting via surface reactivity had no convincing activity, except for CeO 2 NP. Cytotoxicity in differentiated peripheral blood mononuclear cells was the most accurate showing 89% accuracy and 11% false negativity in predicting acute lung inflammogenicity. However, the haemolysis assay showed 100% consistency with the lung inflammation if any dose, having statistical significance was considered positivity. Other cell-based in vitro assays showed a poorer correlation with in vivo inflammogenicity.
Conclusions
Based on the toxicity mechanisms of NPs, two different approaches can be applied for prediction of in vivo lung inflammogenicity. Most in vitro assays were good at detecting NPs that act via soluble ions (i.e., ZnO and CuO NP). However, in vitro assays were limited in detecting NPs acting via surface reactivity as their mechanism of toxicity, except for the haemolysis assay.
Related collections
Most cited references30

- Record: found
- Abstract: found
- Article: found
Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy
- Record: found
- Abstract: not found
- Article: not found
A review of the in vivo and in vitro toxicity of silver and gold particulates: particle attributes and biological mechanisms responsible for the observed toxicity.
- Record: found
- Abstract: found
- Article: not found