- Record: found
- Abstract: found
- Article: found
Efficacy of a Mandibular Advancement Appliance on Sleep Disordered Breathing in Children: A Study Protocol of a Crossover Randomized Controlled Trial
Read this article at
Abstract
Background: Sleep-Disordered Breathing (SDB) varies from habitual snoring to partial or complete obstruction of the upper airway and can be found in up to 10% of children. SDB can significantly affect children's wellbeing, as it can cause growth disorders, educational and behavioral problems, and even life-threatening conditions, such as cardiorespiratory failure. Adenotonsillectomy represents the primary treatment for pediatric SDB where adeno-tonsillar hypertrophy is indicated. For those with craniofacial anomalies, or for whom adenotonsillectomy or other treatment modalities have failed, or surgery is contra-indicated, mandibular advancement splints (MAS) may represent a viable treatment option. Whilst the efficacy of these appliances has been consistently demonstrated in adults, there is little information about their effectiveness in children.
Aims: To determine the efficacy of mandibular advancement appliances for the management of SDB and related health problems in children.
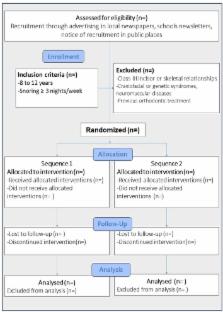
Methods/design: The study will be designed as a single-blind crossover randomized controlled trial with administration of both an “Active MAS” (Twin-block) and a “Sham MAS.” Eligible participants will be children aged 8–12 years whose parents report they snore ≥3 nights per week. Sixteen children will enter the full study after confirming other inclusion criteria, particularly Skeletal class I or class II confirmed by lateral cephalometric radiograph. Each child will be randomly assigned to either a treatment sequence starting with the Active or the Sham MAS. Participants will wear the appliances for 3 weeks separated by a 2-week washout period. For each participant, home-based polysomnographic data will be collected four times; once before and once after each treatment period. The Apnea Hypopnea Index (AHI) will represent the main outcome variable. Secondary outcomes will include, snoring frequency, masseter muscle activity, sleep symptoms, quality of life, daytime sleepiness, children behavior, and nocturnal enuresis. In addition, blood samples will be collected to assess growth hormone changes.
Trial registration: This study was registered in the Australian New Zealand Clinical Trials Registry (ANZCTR): [ACTRN12614001013651].
Related collections
Most cited references74
- Record: found
- Abstract: found
- Article: not found
Diagnosis and management of childhood obstructive sleep apnea syndrome.
- Record: found
- Abstract: found
- Article: not found
Epidemiology of pediatric obstructive sleep apnea.
- Record: found
- Abstract: found
- Article: not found