- Record: found
- Abstract: found
- Article: found
RNAi-mediated knockdown of MTNR1B without disrupting the effects of melatonin on apoptosis and cell cycle in bovine granulose cells
Read this article at
Abstract
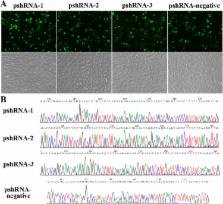
Melatonin is well known as a powerful free radical scavenger and exhibits the ability to prevent cell apoptosis. In the present study, we investigated the role of melatonin and its receptor MTNR1B in regulating the function of bovine granulosa cells (GCs) and hypothesized the involvement of MTNR1B in mediating the effect of melatonin on GCs. Our results showed that MTNR1B knockdown significantly promoted GCs apoptosis but did not affect the cell cycle. These results were further verified by increasing the expression of pro-apoptosis genes ( BAX and CASP3), decreasing expression of the anti-apoptosis genes ( BCL2 and BCL-XL) and anti-oxidant genes ( SOD1 and GPX4) without affecting cell cycle factors ( CCND1, CCNE1 and CDKN1A) and TP53. In addition, MTNR1B knockdown did not disrupt the effects of melatonin in suppressing the GCs apoptosis or blocking the cell cycle. Moreover, MTNR1B knockdown did not affect the role of melatonin in increasing BCL2, BCL-XL, and CDKN1A expression, or decreasing BAX, CASP3, TP53, CCND1 and CCNE1 expression. The expression of MTNR1A was upregulated after MTNR1B knockdown, and melatonin promoted MTNR1A expression with or without MTNR1B knockdown. However, despite melatonin supplementation, the expression of SOD1 and GPX4 was still suppressed after MTNR1B knockdown. In conclusion, these findings indicate that melatonin and MTNR1B are involved in BCL2 family and CASP3-dependent apoptotic pathways in bovine GCs. MTNR1A and MTNR1B may coordinate the work of medicating the appropriate melatonin responses to GCs.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death.
- Record: found
- Abstract: found
- Article: not found
Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes.
- Record: found
- Abstract: found
- Article: not found