- Record: found
- Abstract: found
- Article: found
Five-Year Clinical Outcomes after Neoadjuvant Nivolumab in Resectable Non–Small Cell Lung Cancer
Read this article at
Abstract
Purpose:
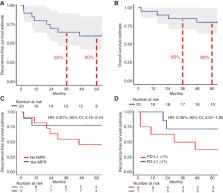
Neoadjuvant anti–PD-1 therapy has shown promise for resectable non–small cell lung cancer (NSCLC). We reported the first phase I/II trial of neoadjuvant nivolumab in resectable NSCLC, finding it to be safe and feasible with encouraging major pathological responses (MPR). We now present 5-year clinical outcomes from this trial, representing to our knowledge, the longest follow-up data for neoadjuvant anti–PD-1 in any cancer type.
Patients and Methods:
Two doses of nivolumab (3 mg/kg) were administered for 4 weeks before surgery to 21 patients with Stage I–IIIA NSCLC. 5-year recurrence-free survival (RFS), overall survival (OS), and associations with MPR and PD-L1, were evaluated.
Results:
With a median follow-up of 63 months, 5-year RFS and OS rates were 60% and 80%, respectively. The presence of MPR and pre-treatment tumor PD-L1 positivity (TPS ≥1%) each trended toward favorable RFS; HR, 0.61 [95% confidence interval (CI), 0.15–2.44] and HR, 0.36 (95% CI, 0.07–1.85), respectively. At 5-year follow-up, 8 of 9 (89%) patients with MPR were alive and disease-free. There were no cancer-related deaths among patients with MPR. In contrast, 6/11 patients without MPR experienced tumor relapse, and 3 died.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries
- Record: found
- Abstract: found
- Article: not found
The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer.
- Record: found
- Abstract: found
- Article: not found