- Record: found
- Abstract: found
- Article: found
Human In Silico Drug Trials Demonstrate Higher Accuracy than Animal Models in Predicting Clinical Pro-Arrhythmic Cardiotoxicity
Read this article at
Abstract
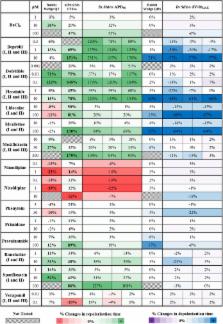
Early prediction of cardiotoxicity is critical for drug development. Current animal models raise ethical and translational questions, and have limited accuracy in clinical risk prediction. Human-based computer models constitute a fast, cheap and potentially effective alternative to experimental assays, also facilitating translation to human. Key challenges include consideration of inter-cellular variability in drug responses and integration of computational and experimental methods in safety pharmacology. Our aim is to evaluate the ability of in silico drug trials in populations of human action potential (AP) models to predict clinical risk of drug-induced arrhythmias based on ion channel information, and to compare simulation results against experimental assays commonly used for drug testing. A control population of 1,213 human ventricular AP models in agreement with experimental recordings was constructed. In silico drug trials were performed for 62 reference compounds at multiple concentrations, using pore-block drug models (IC 50/Hill coefficient). Drug-induced changes in AP biomarkers were quantified, together with occurrence of repolarization/depolarization abnormalities. Simulation results were used to predict clinical risk based on reports of Torsade de Pointes arrhythmias, and further evaluated in a subset of compounds through comparison with electrocardiograms from rabbit wedge preparations and Ca 2+-transient recordings in human induced pluripotent stem cell-derived cardiomyocytes (hiPS-CMs). Drug-induced changes in silico vary in magnitude depending on the specific ionic profile of each model in the population, thus allowing to identify cell sub-populations at higher risk of developing abnormal AP phenotypes. Models with low repolarization reserve (increased Ca 2+/late Na + currents and Na +/Ca 2+-exchanger, reduced Na +/K +-pump) are highly vulnerable to drug-induced repolarization abnormalities, while those with reduced inward current density (fast/late Na + and Ca 2+ currents) exhibit high susceptibility to depolarization abnormalities. Repolarization abnormalities in silico predict clinical risk for all compounds with 89% accuracy. Drug-induced changes in biomarkers are in overall agreement across different assays: in silico AP duration changes reflect the ones observed in rabbit QT interval and hiPS-CMs Ca 2+-transient, and simulated upstroke velocity captures variations in rabbit QRS complex. Our results demonstrate that human in silico drug trials constitute a powerful methodology for prediction of clinical pro-arrhythmic cardiotoxicity, ready for integration in the existing drug safety assessment pipelines.
Related collections
Most cited references46
- Record: found
- Abstract: not found
- Article: not found
SUNDIALS
- Record: found
- Abstract: found
- Article: not found