- Record: found
- Abstract: found
- Article: found
Fucosylated oligosaccharides in mother’s milk alleviate the effects of caesarean birth on infant gut microbiota
Read this article at
Abstract
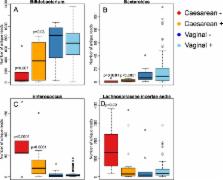
One of the most abundant components in human milk is formed by oligosaccharides, which are poorly digested by the infant. The oligosaccharide composition of breast milk varies between mothers, and is dependent on maternal secretor (FUT2) genotype. Secretor mothers produce milk containing α1-2 fucosylated human milk oligosaccharides, which are absent in the milk of non-secretor mothers. Several strains of bacteria in the infant gut have the capacity to utilise human milk oligosaccharides (HMOs). Here we investigate the differences in infant gut microbiota composition between secretor (N = 76) and non-secretor (N = 15) mothers, taking into account birth mode. In the vaginally born infants, maternal secretor status was not associated with microbiota composition. In the caesarean-born, however, many of the caesarean-associated microbiota patterns were more pronounced among the infants of non-secretor mothers compared to those of secretor mothers. Particularly bifidobacteria were strongly depleted and enterococci increased among the caesarean-born infants of non-secretor mothers. Furthermore, Akkermansia was increased in the section-born infants of secretor mothers, supporting the suggestion that this organism may degrade HMOs. The results indicate that maternal secretor status may be particularly influential in infants with compromised microbiota development, and that these infants could benefit from corrective supplementation.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection.

- Record: found
- Abstract: found
- Article: found
Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants
- Record: found
- Abstract: found
- Article: not found