- Record: found
- Abstract: found
- Article: not found
Coupling Modes and Stoichiometry of Cl −/HCO 3 − Exchange by slc26a3 and slc26a6
Read this article at
Abstract
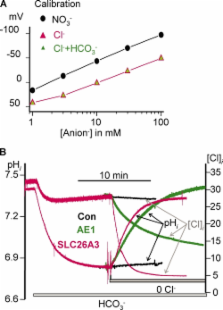
The SLC26 transporters are a family of mostly luminal Cl − and HCO 3 − transporters. The transport mechanism and the Cl −/HCO 3 − stoichiometry are not known for any member of the family. To address these questions, we simultaneously measured the HCO 3 − and Cl − fluxes and the current or membrane potential of slc26a3 and slc26a6 expressed in Xenopus laevis oocytes and the current of the transporters expressed in human embryonic kidney 293 cells. slc26a3 mediates a coupled 2Cl −/1HCO 3 − exchanger. The membrane potential modulated the apparent affinity for extracellular Cl − of Cl −/HCO 3 − exchange by slc26a3. Interestingly, the replacement of Cl − with NO 3 − or SCN − uncoupled the transport, with large NO 3 − and SCN − currents and low HCO 3 − transport. An apparent uncoupled current was also developed during the incubation of slc26a3-expressing oocytes in HCO 3 −-buffered Cl −-free media. These findings were used to develop a turnover cycle for Cl − and HCO 3 − transport by slc26a3. Cl − and HCO 3 − flux measurements revealed that slc26a6 mediates a 1Cl −/2HCO 3 − exchange. Accordingly, holding the membrane potential at 40 and −100 mV accelerated and inhibited, respectively, Cl −-mediated HCO 3 − influx, and holding the membrane potential at −100 mV increased HCO 3 −-mediated Cl − influx. These findings indicate that slc26a6 functions as a coupled 1Cl −/2HCO 3 − exchanger. The significance of isoform-specific Cl − and HCO 3 − transport stoichiometry by slc26a3 and slc26a6 is discussed in the context of diseases of epithelial Cl − absorption and HCO 3 − secretion.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS).
- Record: found
- Abstract: found
- Article: not found
