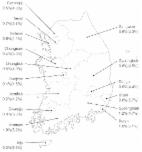
PREAMBLE Aims The Korean Association for the Study of the Liver (KASL) Practice Guidelines for Management of Hepatitis C were first established in 2004, and revised in 2013, when direct-acting antivirals (DAA) were not approved in Korea. Since then, numerous studies of the efficacy, adverse effects and drug-drug interactions (DDI) of interferon-free DAA combination therapy have been published. With all oral DAA therapy showing a sustained virologic response (SVR) rate of 80-90% with minimal adverse events, hepatitis C virus (HCV) eradication is a realistic goal of treatment. Therefore, a screening strategy for HCV-infected populations according to each country’s epidemiology and disease burden is required. DAA combination therapeutics were approved and adapted to practice in Korea in 2015, and KASL revised the guidelines based on a systematic approach that reflects evidence-based medicine and expert opinions. The clinical practice guidelines for the management of hepatitis C have been revised to be useful for treatment, research and education. These recommendations are not absolute standards of care, and adoption of the guidelines in clinical practice may differ among individual patients. Target population The target groups of these guidelines are newly or previously diagnosed patients with hepatitis C virus (HCV) infection, including not only chronic hepatitis C and cirrhosis but also acute hepatitis C patients, hepatitis C patients with chronic kidney diseases, and those patients coinfected with human immunodeficiency virus (HIV) or hepatitis B virus (HBV). Intended users The guidelines are intended to provide useful information and guidance to physicians and healthcare providers involved in the diagnosis and treatment of hepatitis C, and resident physicians, practitioners, and trainers. Development, funding, and revision process The Clinical Practice Guidelines Committee for the Management of Hepatitis C (Committee) comprising 14 hepatologists, was organized according to the proposal and approval of the KASL Board of Executives. Funding for the revision was provided by KASL. Each committee member collected and analyzed the source data in his or her own field, and the members then wrote the manuscript together. Literature review for evidence collection The committee systematically collected and reviewed the international and domestic literature published in PubMed, MEDLINE, KoreaMed, and other databases. The key words used were ‘hepatitis C virus’, ‘hepatitis C’, ‘liver cirrhosis’, ‘liver cancer’ and other related specific key words. Levels of evidence and grades of recommendations The quality of evidence was classified according to the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system (Table 1) [1]. Based on the types of study, randomized control studies were approached from a high level of evidence, while observational studies were approached from a low level of evidence. Then, the level of evidence was adjusted by accounting for the factors influencing the quality of the studies. Through follow-up studies, the level of evidence was defined as follows: A, the highest level of evidence with the smallest possibility of changes in the conclusion; B, a moderate level of potential changes; and C, the lowest level of evidence with the greatest possibility of changes. The strength of a recommendation was also classified according to the GRADE system. Each study was classified as strong recommendation (1) or weak recommendation (2) based on the quality of evidence, the balance between the desirable and undesirable effect of an intervention, and socioeconomic aspects including cost or availability. A strong recommendation indicated that the interventions could be applied in most patients with strong certainty and that there was a greater possibility of desirable effects, high-quality evidence, and presumed patient-important outcomes, cost-effectiveness, preference, and compliance. A weak recommendation indicated a suggestion made with less certainty but that could be considered favorable for many patients, based on the level of evidence, the cost or preferences of the patients or medical practitioners. List of key questions The revision committee considered the following clinical questions as the key components to be covered in these guidelines. 1. What is the epidemiology, natural history and prevention strategy of hepatitis C in Korea? 2. How should diagnosis and evaluation of severity of chronic hepatitis C be made? 3. What is the goal of treatment and who are the targets for antiviral treatment of hepatitis C? 4. How to define the treatment response and what are the predictors of the response? 5. How to treat patients with genotype 1 chronic hepatitis C and compensated cirrhosis? 6. How to treat patients with genotype 2 chronic hepatitis C and compensated cirrhosis? 7. How to treat patients with genotype 3 chronic hepatitis C and compensated cirrhosis? 8. How to treat patients with genotype 4 chronic hepatitis C and compensated cirrhosis? 9. How to treat patients with genotypes 5 and 6 chronic hepatitis C and compensated cirrhosis? 10. How to treat patients with decompensated cirrhosis? 11. How to treat patients who underwent liver or extrahepatic organ transplantation? 12. How to treat patients with acute hepatitis C? 13. How to monitor the patients and the adverse effects of antiviral drugs during and after antiviral treatment? 14. How to treat patients with special conditions (people who inject drugs, chronic kidney diseases, coinfection with HIV or HBV, hemophilia or thalassemia, immunosuppressive therapy or cytotoxic chemotherapy, and pediatric patients)? Review of the manuscript and approval process Each manuscript written by committee members was reviewed, agreed, and approved through meetings of the committee. The quality of the manuscript was evaluated based on the standards suggested by AGREE II (Appraisal of Guidelines for Research and Evaluation II) along with the academic integrity of the contents. The guidelines were reviewed after counsel from an infection specialist, at a meeting of an external review board composed of 14 KASL members, and were further modified following opinions aired at a public hearing, and a symposium open to all KASL members. The final manuscript was approved by the KASL Board of Executives. Release of the guidelines and plan for updates The Korean version of the KASL Clinical Practice Guidelines for the Management of Hepatitis C was released in November 2015 at a KASL meeting and published in January 2016 on the KASL website (http://www.kasl.org). Future plans for revision will be conducted under the judgment that the revision is necessary for promotion of health in South Korea with accumulation of research on the management of hepatitis C. In addition, use of DAA is to be allowed in South Korea in the near future, so that updating or partial revision of the guidelines, as appropriate, is warranted. EPIDEMIOLOGY HCV is one of the main causes of acute and chronic hepatitis, cirrhosis, and hepatocellular carcinoma [2]. Hepatitis C is on the list of National Notifiable Infectious Disease in South Korea and has been under surveillance since 2000. An effective HCV vaccine is yet to be discovered, so that understanding of national epidemiology and a preventive strategy to block routes of HCV infection is important for public health. Prevalence of HCV infection The worldwide prevalence of HCV infection was 2.8% in 2005 and 1.6% in 2014, equating to about 115 million persons positive for antibody to hepatitis C (anti-HCV) and 1.1% equating to about 80 million persons positive for HCV RNA [3]. The prevalence varies among geographic regions; regions with a high prevalence of over 3.5% include Central Asia (including Mongolia and China), SouthEast Asia (including Pakistan and Thailand), and North Africa (including Egypt). Low prevalence (below 1.5%) regions are Asia (including South Korea and Japan), North America (including the United States), and South America [4]. 1. Prevalence in adult health check examinees The prevalace of HCV infection in adult health check examinees was reported to be 1.7% using a first-generation enzyme immunoassay (EIA) in the early 1990s soon after HCV was discovered [5]. The estimated age-standardized prevalence of anti-HCV in adult health check examinees in > 40 years of age was reported to be 1.29% (95% confidence interval, 1.12-1.48) and was over 193,000 persons in a collective study of health checkup examinees from Seoul, Ulsan, Jeollanam-do, and Daegu between 1995 and 2000 [6-10]. In 2009, the anti-HCV prevalence in health examinations of 291,314 adults ≥20 years of age from 29 health examination centers was 0.78% using third-generation EIA after adjusting for age, sex, and area [11]. The anti-HCV prevalence was higher in females (0.83%) than in males (0.75%) and increased with age (20-29 years: 0.34%, 30-39 years: 0.41%, 40-49 years: 0.60%, 50-59 years: 0.80%, 60-69 years: 1.53%, ≥70 years: 2.31%). In addition, the anti-HCV prevalence varied geographically; in comparison with the prevalence of 0.50-1.20% in most regions, including Seoul and Gyeonggi-do, the prevalence in Pusan and Jeollanamdo were 1.53% and 2.07%, respectively, while the Jeju Special Self-Governing Province had the lowest rate of 0.23% (Fig. 1). And also, there was a significant geographic difference in the potential risk factors for HCV infection in Korea [12]. An update of the national HCV infection prevalence in South Korea is expected to be released at the end of 2015, and the Korea National Health and Nutrition Examination Survey has included anti-HCV testing since 2012. 2. Prevalence of anti-HCV in blood donors, pregnant women, and children The anti-HCV prevalence in 2,040,151 blood donors in 1997 in South Korea was 0.34%, as determined by third-generation EIA [13]. From 2005 to 2009, the anti-HCV prevalence in 11,064,532 blood donors was 0.16%, and the HCV RNA-positive rate was 8.4 (0.0084%) of 100,000 donors, among whom 81% were young people aged 10-30 years [14]. In South Korea, the risk of blood transfusion-related HCV infection decreased from 1 in 81,431 in 2000/2001 to 1 in 2,984,415 after implementation of nucleic acid testing for HCV screening of donated blood in February 2005 [15]. The anti-HCV prevalence in pregnant women was reported to be 0.49-1.7% [16-18], and a domestic report investigating over 5,000 pregnant women reported rates of 0.42-0.44% [19,20]. Among anti-HCV-positive pregnant women, 57-60% of the patients positive for HCV RNA [19,20]. Domestic studies on anti-HCV prevalence in children and adolescents are insufficient. A 0.82% anti-HCV-positive rate tested by third-generation EIA in 2,080 children between 6 and 11 years of age living in Seoul was reported [21]. However there have been no other reports or studies on children and adolescents, hindering the accurate assessment of the HCV infection prevalence in the pediatric population of South Korea. 3. Prevalence of anti-HCV in high-risk groups The high-risk groups for HCV infection include people who inject drugs (PWID), patients under hemodialysis, and those with HIV infection, hemophilia, and leprosy. However, the HCV prevalences in this group have been reported mostly before 2000; few studies were performed thereafter. The domestic anti-HCV prevalence in the intravenous drug user (IVDU) group was 48.4-79.2% [22-25]. Among anti-HCV-positive persons, 98.1% were HCV RNA-positive [24]. The anti-HCV prevalence in those who share cocaine suction pipes was similar to that in the IVDU group [26]. The anti-HCV prevalence was 5.9-14.7% in previous studies of >200 patients with chronic kidney diseases [27,28], 2.2% in the 2014 report of the Korean Society of Nephrology [29] and was significantly correlated with hemodialysis duration. The HCV coinfection rate was high in those infected with HIV; ~25% of westerners and 5.0-6.3% of HIV-infected individuals in South Korea were coinfected with HCV [30-32]. The anti-HCV prevalence in 104 hemophilia patients tested by third-generation EIA was 42.3% in 2002, and the risk of infection was correlated with age and severity of hemophilia [33]. In their 2012 annual report, the Korea Hemophilia Foundation (KHF) reported that 430 of 2,148 (20.0%) hemophilia patients were anti-HCV-positive and 118 of 2,148 (5.5%) were HCV RNA-positive [34]. Leprosy patients can be considered at high risk of HCV infection due to their skin lesions and long-term cohabitation in limited areas. The anti-HCV prevalence of 96 leprosy patients tested by second-generation EIA was 67.7% in 1997; 82% of these individuals were immunoblot-positive [35]. HCV incidence rate Studies on HCV infection incidence rate are rare, since only 20-30% of those with acute HCV infection develop symptoms. HCV incidence rates are decreasing in Western countries [36]. In the US the incidence rate decreased from 7.4 of 100,000 people from 1982-1989 to 0.7 of 100,000 people from 1994-2006 [37], and in Italy, it decreased from 2.02 of 100,000 people in 1996 to 0.55 of 100,000 people in 2006 [38]. The HCV infection incidence rate in South Korean blood donors was reported to be 13.8 per 100,000 according to a survey conducted on those who donated blood at least twice from 1994-1996 [39]. The recent HCV infection incidence rates among blood donors who donated at least twice in 2 years between 2000 and 2010 were estimated to be 6.80 in 2001, 3.19 in 2003, 2.69 in 2005, 1.83 in 2007, and 0.80 in 2009 per 100,000 person-years, showing significant decrease in the incidence of HCV in this population in South Korea [15]. According to surveillance sample data from the Korea Centers for Disease Control and Prevention (KCDC), the number of reported hepatitis C cases was 1,927 in 2002, 6,407 in 2008, and 4,280 in 2012. Future studies should evaluate the nationwide incidence of HCV in Korea. Distribution of HCV genotypes Globally, HCV genotypes 1, 2, and 3 are common and genotypes 4, 5, and 6 are localized to limited regions [40,41]. Genotype 1a is the most common in Northern Europe and North America, and 1b is the most common in Far East Asia and Europe. Genotype 2 is less common than genotype 1. Genotype 3 is common in Southeast Asia and genotype 4 is common in the Middle East, Egypt, and Central Africa. Genotype 5 is commonly found in South Africa and genotype 6 is common in Hong Kong, Macau, and Vietnam. Common HCV genotypes in South Korea are genotype 1b (45- 59%) and 2a (26-51%); types 1a, 2b, 3, 4, and 6 are rare in South Korea [42,43]. Whether genotype 1 HCV infection provokes faster progression of hepatic disease than the other genotypes is controversial. A recent meta-analysis reported that genotype 1b patients showed a 1.78-fold higher risk of developing HCC (95% CI, 1.36-2.32) compared to non-1 genotype patients [44]. Nevertheless, the HCV genotype is the most crucial factor in determining the efficacy of antiviral therapy [45]. PREVENTION Route of transmission HCV transmission occurs by parenteral exposure. The main routes of transmission include transfusion of contaminated blood or blood products, organ transplantation, PWID, unsafe injection or medical procedures, stabs by contaminated syringe or needle, sexual contact with HCV-infected person, or perinatal transmission from an infected mother to her newborn. Transmission via transfusion was a main route of infection until 1991, but the possibility has become extremely low since introduction of a screening test for blood donors [46-48]. Recently, in developed regions such as the US and Europe which have low HCV prevalence, the most common route of HCV transmission has been the use of illicit drugs [49], and anti-HCV prevalence in the PWID group was reported to be up to 50-90% [50]. Meanwhile, unsafe injection with multiple-use medication vials or reused syringes, or unsanitary medical procedures including surgery, endoscopy, and dental treatment without proper disinfection are the main causes of HCV transmission in developing countries [51-53]. In addition, meta-analyses have reported that risk factors for HCV transmission include piercing, acupuncture, or tattooing without proper disinfection [54-56]. The risk of HCV infection due to percutaneous exposure to a small dose, such as needle sticks, is 1.8% (0-7%) [57-60] in other countries and 0.92% in South Korea [61]. Heterosexual persons with chronic HCV infection in long-term monogamous relationships with a partner had little evidence of sexual transmission of HCV. However, the risk becomes higher with multiple sex partners, and unsafe sex including anal sex, sex accompanying wounds, sex with carriers of other sexually transmitted diseases such as HIV, or in homosexuals [62,63]. The percentage of perinatal transmission was 1-6.2% [64,65]. It was reported to be 1.7% when the mothers were positive for anti-HCV regardless of HCV RNA-positivity, and 4.3% (3.9-7.1%) in the case of HCV RNA-positive mothers [65,66]. The risk of perinatal transmission increased in female infants, HIV-positive mothers, and mothers with high blood HCV RNA levels [67]. Cesarean section reportedly does not prevent HCV transmission [67,68], and the frequency of transmission via nursing was very low. Thus, it is not necessary to limit breast-feeding unless nipples are injured or bleeding [69]. Reports of horizontal transmission between siblings or family members of HCV-infected persons are based on a low level of evidence [70]. A comparative study of 1,173 HCV patients and 534 controls in five university hospitals between 2007 and 2011 in South Korea reported several independent risk factors of infection, including use of illicit drugs, needle-stick injury, transfusion before 1995, tattoo, and age [71]. Counseling for prevention Since an effective vaccine has not been developed, the main strategy for prevention is to educate people on the risk factors for HCV infection and to maintain strict sanitation standards in all locations performing percutaneous procedures. HCV-infected persons should be counseled not to donate blood, organs, tissues, or semen, and not to share any instrument penetrating skin. They should use their own instruments including toothbrushes, oral hygiene devices, razors, or nail clippers so other people are not exposed to his/her blood. Finger-stabbing needles commonly used for Korean home remedies should not be shared. IVDU should be persuaded to stop drug abuse and they should not reuse syringes, needles, injection solution, cotton swab, or alcohol sponges. They must be reminded that other people can be infected via recklessly disposed needles. Since the risk of infection among monogamous couples is very low, use of barrier protection by these couples is not necessarily recommended. Nevertheless, if the partner of the infected individual requests, or if the infected person has multiple sex partners, it is recommended to use condoms. Routine screening for HCV is not recommended for all pregnant women. However, for those with a risk factor, prenatal testing for HCV is required. HCV infection does not mean a restriction of breast-feeding or recommendation for a specific means of delivery, such as Cesarean section. Healthcare facilities should take precautions to prevent HCV transmission. Proper disinfection, cleaning, and management of materials and instruments are essential in medical and invasive procedures including tattooing, piercing, and acupuncture. [Recommendations] 1. HCV-infected persons should not donate blood, organs, tissues, or semen (A1). HCV-infected persons should avoid sharing toothbrushes, oral hygiene devices, razors, nail clippers, or any instrument penetrating skin, so as not to expose other people to his/her blood (C1). 2. People who inject drugs should be counseled to stop abuse of illicit drugs (A1). They should be educated about routes of infection and tested regularly for HCV infection (B1). 3. Proper disinfection, cleaning, and management of materials and instruments are essential in medical and invasive procedures including tattooing, piercing, and acupuncture (B1). 4. As the risk of infection among monogamous sexual partners is very low, use of barrier protection is not advised in these couples (B1). However, for those with multiple sex partners, it is recommended to use condoms (B1). 5. For pregnant women, if a risk factor for HCV infection is detected or HCV infection is suspected otherwise, prenatal testing for HCV infection is recommended (B1). HCV infection does not mean a restriction of breast-feeding or a recommendation of specific delivery, such as Cesarean section (B2). NATURAL HISTORY Acute HCV infection After 1-3 weeks of HCV infection, HCV RNA becomes detectable in blood and the level rapidly increases [72,73]. The serum alanine transaminase (ALT) level increases due to hepatocyte damage after 4-12 weeks of infection. Most infections are asymptomatic (70-80%), but symptoms including flu-like symptoms, fatigue, vomiting, nausea, right upper quadrant pain, muscle pain, or pruritus may develop within 2-12 weeks. About 20% of acute infection accompanies jaundice with serum bilirubin level 90% cure rate of DAA in early stage liver disease, should also be taken into consideration. One-time screening for HCV infection in the South Korean population aged 40-70 years is likely to be more cost-effective compared to current practice [114]. Therefore, a nationwide screening and therapeutic strategy to diagnose and cure hepatitis C patients prior to progression to advanced liver disease would not only decrease the disease burden but also be a successful model for HCV elimination. Diagnosis of HCV infection Biochemical tests, serologic assays, and HCV RNA testing are needed to confirm HCV infection. Physical examination and history taking should be performed to understand the routes of transmission and prevent further reinfection. HCV genotyping is essential for treatment, and radiologic examination, liver biopsy, or noninvasive evaluation of hepatic fibrosis can be performed to determine the necessity of treatment, and to assess liver disease severity. Interpretation of serological and virological test results is summarized in Table 3. DIAGNOSIS Serologic assays: Anti-HCV test Detection of anti-HCV in serum or plasma is used for screening of high-risk groups and for diagnosis of acute or chronic hepatitis C [115]. The third-generation EIA uses recombinant core, NS3, NS4, and NS5 HCV proteins, and its sensitivity and specificity are 97.2- 99% and 99.8-100%, respectively, when tested in immune-competent individuals [116-118]. If signal/cutoff (S/CO) ratios of third-generation EIA exceed 3.8, a positive result will be apparent in 95% of recombinant immunoblot assays (RIBA) [119-121]. However, the cut-off S/CO ratio can differ according to the type of equipment, so that high S/CO ratios do not always mean true positive [122]. Recently, use of enhanced chemiluminescent immunoassay (CLIA) or electrochemiluminescence immunoassay (ECLIA) is increasing since those assays detect antigen-antibody reaction more sensitively compared to the third-generation EIAs. Meanwhile, pointof-care tests using saliva or fingerstick blood that produce results within 20 minutes can also be employed [123,124]. Average time between HCV infection and seroconversion is 8-9 weeks, and anti-HCV is detectable in >97% of patients with HCV infection within 6 months [107,125]. Anti-HCV is not a neutralizing antibody and persists indefinitely in chronic hepatitis C patients and after recovery. Therefore, the differentiation of current from past infection after recovery is impossible using anti-HCV positivity. Anti-HCV can be tested repeatedly in high-risk persons because hepatitis C virus can reinfect after recovery. Negative result for anti-HCV in combination with a positive result for HCV RNA may represent an early stage of acute infection or chronic infection in the setting of severe immunosuppression, such as patients on hemodialysis, HIV coinfection, solid organ transplantation recipients, hypo-/ a-gammaglobulinemia, and patients with HCV-associated essential mixed cryoglobulinemia [126-128]. In these patients, HCV RNA testing is necessary for diagnosis of HCV infection. In contrast, false-positive result for anti-HCV and negative result for HCV RNA can occur in patients with autoimmune diseases [129]. Virological assays 1. HCV RNA assays HCV RNA assays are classified as quantitative and qualitative. Since the detection cutoff of qualitative assays is 50 IU/mL, and these are more sensitive than previous-generation quantitative assays, HCV RNA qualitative assays have been used for diagnostic confirmation of HCV infection, and HCV RNA quantification is used for pretreatment assessment and monitoring of a virological response during and after antiviral therapy [115,121,130]. However, recently available quantitative HCV RNA assays use real-time polymerase chain reaction (PCR) and transcription-mediated amplification (TMA), and are highly sensitive with lower detection limits of 12-15 IU/mL, while they have a broad measuring range with an upper limit of 7-8 log IU/mL and 98-99% diagnostic specificity irrespective of HCV genotype [115,131-135]. Therefore, quantitative HCV RNA tests are now widely used for both diagnosis and evaluation of the treatment response [127,136]. In 1997, the World Health Organization established an international standard for HCV RNA quantification unit, IU, rather than HCV copy number [137,138]. However, since viral quantification results can differ among laboratories [139], it is recommended to use the same laboratory test before, during, and after-treatment for monitoring, if possible [107,127]. Blood HCV RNA is detectable as early as 2 weeks after infection [76], rapidly increases to reach a plateau, and decreases along with ALT after ALT has peaked [140]. HCV RNA levels remain steady in patients with chronic hepatitis C [140,141]. HCV RNA levels are not significantly correlated with the severity of hepatic inflammation or fibrosis, and change little during chronic infection without antiviral treatment [142,143]. 2. Genotyping/subgenotyping assays HCV genotyping is useful for epidemiologic studies as well as for predicting treatment response. Therefore, HCV genotype should be assessed before treatment to determine the optimal therapeutic duration and dose of ribavirin [144]. HCV is classified into six major genotypes (1-6) and is subdivided into subtypes identified by lower-case letters, such as 1a or 1b. Differences of 31-33% at the nucleotide level differentiate the genotypes, compared with 20-25% for the subtypes [145]. HCV genotype does not change within a person unless reinfected. HCV genotypes and subtypes can be determined by direct sequence analysis, reverse hybridization, or restriction fragment mass polymorphism (RFMP) [146]. Most genotyping assays analyze both the 5’-untranslated region (UTR) and HCV core regions, the nucleotide sequences of which are highly conserved [147-150]. Subtyping is not necessary in antiviral therapy using interferon alpha and ribavirin, but in treatment that includes DAAs, subtypes may need to be confirmed since DAAs act differently according to subgenotype [148-153]. Genotyping is not possible in 1.5 (AUROC=0.8) and cirrhosis defined as APRI>2 (AUROC=0.89) [214]. A normal value of AAR is 1.0 has a 73.7-100% positive predictive value for diagnosis [207,215-217]. FIB-4 is calculated using the formula: age (yr) × AST (IU/L)/platelet count (109/L × [ALT (IU/L)1/2] (www.kasl.org). A FIB-4 score 3.25 has a positive predictive value of 65% for advanced fibrosis [218]. Liver stiffness measurement using transient elastography can be used to assess hepatic fibrosis [219-222]. However, transient elastography cannot totally replace liver biopsy, because it often cannot produce reliable measurements in obese patients, and tends to give falsely high results in cases of acute hepatitis with severe inflammation and necrosis with mild fibrosis [223,224]. In the case of chronic hepatitis C, cutoff values determining significant fibrosis (≥F2) vary among studies, ranging from 7.1 to 8.8 kPa, with an AUROC of 0.79-0.83 [225]. The AUROC for diagnosis of liver cirrhosis ranged from 0.95-0.97, with cutoff values of 12.5-14.6 kPa (77-78% positive predictive value, 95-97% negative predictive value) [202,209,219,225-228]. Other newly developed noninvasive tests include acoustic radiation force impulse (ARFI) imaging, real-time elastography, magnetic resonance (MR) elastography, diffusion-weighted MR image, and MR spectroscopy. However, their effectiveness remains to be validated [229-231]. [Recommendations] 1. Screening for HCV infection could be considered in populations at risk as well as those over 40 years old with increasing prevalence of HCV infection (C1). 2. Anti-HCV should be tested in patients suspected of having acute or chronic HCV infection (A1). 3. HCV RNA should be tested in patients with a positive anti-HCV test to confirm the diagnosis (A1). 4. Even if anti-HCV is negative, HCV RNA testing is required when acute HCV infection is suspected or in the presence of unexplained liver disease in immunosuppressed patients (B1). 5. HCV RNA quantitative assay and genotyping/subgenotyping (1a/1b) should be performed prior to antiviral treatment (A1). 6. Immediately following exposure to infected blood or body fluids, anti-HCV and serum ALT level testing should be performed. If anti-HCV is negative, an HCV RNA assay should be conducted 4-6 weeks after exposure for early diagnosis. If all baseline tests are negative, follow-up testing for anti-HCV and serum ALT level should be performed 4-6 months after the exposure (B2). 7. Assessment of liver disease severity is essential prior to antiviral treatment (A1). 8. Liver biopsy and/or noninvasive tests for assessment of hepatic fibrosis can be performed to make treatment decision and predict prognosis (B1). TREATMENT GOALS The goals of hepatitis C treatment are to eradicate HCV and to prevent complications of liver cirrhosis, hepatocellular carcinoma, extrahepatic manifestations of HCV infection and death. It is difficult to evaluate the treatment goal in a short period of time due to the gradual progression of chronic hepatitis C over several decades. Therefore, the short-term goal of hepatitis C treatment is to achieve an SVR, defined as undetectable serum HCV RNA by a sensitive assay 12 or 24 weeks after the end of treatment. SVR was determined 24 weeks after the end of treatment using an assay with a lower limit of detection of 800,000 IU/mL [250,263,266]. SVR rates are lower in older patients (>40 years) [254], African-Americans [267], body weight >70 kg [254,261], and insulin resistance [268,269]. SVR rates vary depending on SNP of IL28B, in other words, a C or T allele at the rs12979869 locus [270]. The SVR rates of HCV genotype 1 Caucasian patients are 69%, 33%, and 27% in CC homozygotes, CT heterozygotes, and TT homozygotes, respectively. The SVR rates of HCV genotype 1 African-American patients are 48%, 15%, and 13% [263,271]. The SVR rates of HCV genotype 1 Korean patients are 73-88% in CC homozygotes and 0-40% in CT heterozygotes [272-274]. The prevalence of the IL28B genetic polymorphism varies among ethnicities. CC homozygotes in Korea account for 88-89% [272-274], compared to 17% in African-Americans, and 37% in Caucasians [263]. Therefore, the usefulness of the IL28B genetic polymorphism as a predictive factor is limited as over 90% of the population of Korea are CC homozygotes. During combination treatment with PegIFN-α and ribavirin, a RVR is the strongest on-treatment predictor for an SVR [250-252], and an SVR rate increases ninefold with a RVR [263]. Meanwhile, an EVR is a strong negative predictor for an SVR. Without EVR, the SVR rate is only 3% [255]. In addition, SVR rates increase when medication adherence is higher than 80% [275], so assessment and maintenance of medication adherence can increase the SVR rate. NEW DRUGS, DIRECT-ACTING ANTIVIRALS (DAA) DAA acts at a specific step of the viral life cycle. DAAs include NS3/4A protease inhibitors (PI), NS5A inhibitors, and NS5B polymerase inhibitors. NS3/4A PI is a first-generation DAA and blocks the polyprotein processing essential for HCV replication. To date, available drugs are boceprevir, telaprevir, simeprevir, asunaprevir, and paritaprevir. NS5A inhibitors, such as daclatasvir, ledipasvir or ombitasvir, affect various HCV genotypes and show a synergistic effect in combination with other DAAs. The NS5B polymerase inhibitors are divided into nucleoside polymerase inhibitors (sofosbuvir) and non-nucleoside polymerase inhibitors (dasabuvir, beclabuvir). Because the basic characteristics of each DDA differ, selection and use of the appropriate drugs should take into consideration hepatic and renal function. DAA regimens may have a risk of interactions with other medications used by patients. Prior to starting treatment, patients should be evaluated for potential drug-drug interactions with selected DAAs. A more comprehensive list of drug-drug interactions is available at several websites, such as www.hep-druginteractions.org. [Recommendations] 1. The characteristics of the DAAs should be understood, and the appropriate drugs selected taking into consideration hepatic and renal function (A1). 2. The potential for drug-drug interactions must be considered before and during treatment with DAAs. The full prescribing information must be consulted prior to use of DAAs due to the potential for drug-drug interactions (A1). Simeprevir Simeprevir is a HCV NS3/4A protease inhibitor. 1. Dosage and administration One 150 mg capsule is taken orally once daily with food. 2. Pharmacokinetics Simeprevir primarily undergoes oxidative metabolism by the hepatic CYP3A system. Elimination of simeprevir occurs via biliary excretion. Renal clearance plays an insignificant role in its elimination. Therefore, no dose adjustment of simeprevir is needed in patients with mild, moderate or severe renal impairment. Compared to HCV-uninfected subjects with normal hepatic function, the mean steady-state AUC of simeprevir was higher in HCV-uninfected subjects with moderate hepatic impairment (Child-Pugh Class B) and severe hepatic impairment (Child-Pugh Class C). Simeprevir has not been extensively studied in such patients, but has been used in real-life settings. 3. Drug-drug interactions Co-administration of simeprevir with substances that are moderate or strong inducers or inhibitors of CYP3A4 is not recommended as this may lead to significantly lower or higher exposure to simeprevir, respectively. A number of compounds are contraindicated in patients receiving simeprevir, including anticonvulsants (carbamazepine, phenobarbital, phenytoin), antibiotics (erythromycin, clarithromycin), antimycobacterials (rifampin, rifabutin, rifapentine), antifungals (itraconazole, ketoconazole, fluconazole, voriconazole), dexamethasone, cisapride, herbal products (milk thistle, St John’s wort) and antiretroviral drugs (cobicistat-based regimens, efavirenz, etravirine, nevirapine, ritonavir). Simeprevir does not require dose changes in combination with the immunosuppressants tacrolimus and sirolimus. In contrast, it is not recommended to co-administer simeprevir with cyclosporine, as this can result in significantly increased plasma concentrations of simeprevir. Dose adjustments are needed with some antiarrhythmics, warfarin, calcium-channel blockers, HMG Co-A reductase inhibitors and sedative/anxiolytics. 4. Adverse reactions and safety Most common adverse reactions in patients receiving simeprevir, PegIFN-α and ribavirin were rash (including photosensitivity), pruritus and nausea. Transient hyperbilirubinemia was observed, but it was not associated with elevations in liver transaminase levels. Asunaprevir Asunaprevir is a HCV NS3/4A protease inhibitor. 1. Dosage and administration One 100 mg capsule is taken orally twice daily with or without food. 2. Pharmacokinetics Asunaprevir undergoes oxidative metabolism primarily mediated by CYP3A. Following single-dose oral administration in healthy subjects, 84% of it was recovered in feces and less than 1% was recovered in the urine. No dosage adjustment is required for patients with mild and moderated renal impairment (eGFR 30-80 mL/min/1.73 m2). Dosage adjustment of asunaprevir to 100mg once daily is recommended for patients with severe renal impairment (eGFR 75 kg. PegIFN-α 2b is to be injected 1.5 μg/kg/week with ribavirin using doses of 800 mg for those 105 kg. In patients with a RVR and low baseline HCV viral load ( 75 kg. PegIFN-α 2b is to be injected 1.5 μg/kg/week with ribavirin using doses of 800 mg for those 105 kg (A1). TREATMENT OF CHRONIC HCV GENOTYPE 5 OR 6 INFECTION There is no report of HCV genotype 5 in South Korea, which is limited mostly to South Africa. HCV genotype 6 is limited mostly to Southeast Asia, Southern China, Hong Kong, and Macau. It comprises about 1% of total chronic HCV patients in South Korea [352]. SVR rate of chronic HCV genotype 5 or 6 treated with a combination of PegIFN-α and ribavirin is 70-86%, which is comparable with that of HCV genotype 3 and higher than that of HCV genotype 1 [353-355]. Studies for HCV genotype 5 or 6 are limited because of the small number of patients infected with HCV genotype 5 or 6. The combination of ledipasvir/sofosbuvir for 12 weeks as an IFN-free DAA regimen showed a 96% SVR in 25 treatment-naïve and -experienced patients infected with HCV genotype 6 [340]. This result can be extrapolated for treatment of HCV genotype 5, although there are no data regarding use of this combination in patients infected with HCV genotype 5, because ledipasvir is active against both genotypes 5 and 6 in vitro. Treatment with PegIFN-α, weight-based ribavirin and sofosbuvir for 12 weeks achieved a 100% SVR in one patient infected with HCV genotype 5 and six patients infected with HCV genotype 6 [259]. Due to the weak evidence, the results of several trials involving patients infected with genotype 1 can be extrapolated. IFN-free regimens for the treatment of cirrhotic patients infected with HCV genotype 5 or 6 could be prolonged to 24 weeks or weight-based ribavirin added to improve the SVR rate. If DAAs are not available, the previous standard therapy such as the combination of PegIFN-α and ribavirin for 24 weeks remains acceptable. [Recommendations] (Table 10) Initial treatment of HCV genotype 5 or 6 1. Patients infected with HCV genotype 5 or 6 without cirrhosis can be treated with daily ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks (B1). Duration of therapy can be extended to 24 weeks or daily weight-based ribavirin (1,200 mg in patients ≥ 75 kg, 1,000 mg in patients 75 kg. PegIFN-α 2b is to be injected 1.5 μg/kg/week with ribavirin using doses of 800 mg for those 105 kg (A1). TREATMENT OF PATIENTS WITH DECOMPENSATED CIRRHOSIS In the SOLAR-2 study, which was a multicenter randomized controlled trial of 108 patients with HCV genotypes 1 and 4 with decompensated cirrhosis (Child-Pugh Turcotte [CTP] class B or C, CTP scores ≤ 12), participants were randomly assigned to receive daily fixed-dose combination ledipasvir (90 mg) and sofosbuvir (400 mg) and ribavirin (initial dose of 600 mg, increased as tolerated) for 12 or 24 weeks [356]. SVR was achieved in 87% and 89% of patients given the 12- and 24-week treatment courses, respectively. Baseline CTP and Model for End-Stage Liver Disease (MELD) scores improved in more than 50% of the patients, but some patients experienced worsening of hepatic function. During the treatment, five (5%) patients died of variceal bleeding. Grade 3 or 4 adverse events occurred in 15% and 34% in the 12- and 24-week arms, respectively. In the phase III ALLY-1 study, daclatasvir (60 mg) was administered daily in combination with sofosbuvir (400 mg) and a low initial dose of ribavirin (600 mg) for 12 weeks to 60 patients with decompensated cirrhosis (mostly CTP class B and C, HCV genotype 1:3:2/4/6=45:6:9) [357]. The overall SVR rate was 83%. SVR rates were 76% and 100% among patients with HCV genotypes 1a and 1b, respectively. In patients with HCV genotype 1, SVR rates were 92% and 50% among patients with CTP classes B and C, respectively. Among subjects with HCV genotype 3 and 2/4/6, SVR12 rates were 83% and 89%, respectively. The efficacy and safety of simeprevir plus sofosbuvir with or without ribavirin for 12 weeks were assessed in patients with CTP class B/C (n=55) versus CTP class A (n=101) cirrhosis and compared to matched untreated controls [358]. SVR was achieved by 73% of CTP class B/C versus 91% of CTP class A (P 75 kg]) for either 12 weeks or 24 weeks [359]. In patients with Metavir fibrosis stages F0 to F3, SVR was achieved in 96% and 98% of patients in the 12- and 24-week arms, respectively. In patients with compensated cirrhosis, SVR was achieved in 96% of patients in both the 12- and 24-week arms. Efficacy was lower in patients with CTP class B cirrhosis (SVR 85% vs. 88% in the 12- and 24-week arms) or CTP class C cirrhosis (60% vs. 75% in the 12- and 24-week arms). A total of 123 patients infected with HCV genotype 1 received simeprevir and sofosbuvir with or without ribavirin treatment for 12 weeks [365]. An SVR was achieved in 90% of patients, and the addition of ribavirin did not impact the SVR rate. Because of significantly increased plasma concentrations of simeprevir, the concomitant use of simeprevir and cyclosporine is not recommended in liver transplant recipients. No simeprevir dose changes are required with tacrolimus and sirolimus. In the phase III ALLY-1 study, daclatasvir (60 mg daily) was administered in combination with daily sofosbuvir (400 mg) and ribavirin (initial dose, 600 mg) for 12 weeks to patients with recurrent HCV infection post-transplant (n=53, HCV genotype 1:3:2/4/6=41:11:1) [360]. The SVR rate was 94%. SVR rates were 95%, 91% and 100% in patients infected with genotypes 1, 3 and 2/4/6, respectively. In a multicenter study of 34 liver transplant recipients with mild recurrence (Metavir fibrosis stage F0-F2) of HCV genotype 1 infection, fixed-dose combination paritaprevir (150 mg), ritonavir (100 mg), and ombitasvir (25 mg) plus twice-daily dosed dasabuvir (250 mg) and weight-based ribavirin was given for 24 weeks, and achieved an SVR rate of 97% [366]. Two patients (6%) had serious adverse events, and there were no deaths during the study. Because of interactions with ritonavir and paritaprevir, adjustments of the cyclosporine and tacrolimus dose were needed. When interferon-containing treatment is considered in patients with HCV reinfection following liver transplantation, it is recommended after histological confirmation of chronic hepatitis C at least 6 months after transplantation. This is because shortly after transplantation, patients are heavily immunosuppressed and incompletely recovered from the surgery, resulting in a high probability of drug intolerability as well as allograft rejection during interferon use. The SVR rate of interferon-containing antiviral treatment after transplantation is 30-40%, and genotypes 2 and 3 show better therapeutic outcomes than genotype 1 [245,367,368]. Post-transplant patients reportedly show similar therapeutic outcomes (33% and 38% SVR rates, respectively) using PegIFN-α plus ribavirin combination therapy or PegIFN-α monotherapy, which may be due to frequent dose reduction or discontinuation of ribavirin due to complications [369]. Anemia is the most common cause of treatment discontinuation and recombinant erythropoietin is recommended in this case [367,368]. Allograft rejection related to interferon alpha use can occur, and liver biopsy is required to differentiate the cause of liver function deterioration during antiviral treatment. Treatment following other organ transplants Renal transplant patients with HCV infection display rapidly progressing hepatic fibrosis and show high mortality related to hepatic failure; thus, antiviral treatment was recommended prior to renal transplantation in the past [370]. However, with the introduction of DAAs, successful elimination of HCV after renal transplantation could be achieved. It remains to be determined whether patients with chronic hepatitis C should optimally proceed to renal transplantation, with the expectation that their hepatitis C can be cured post-transplant to improve the outcome. Combination therapy with PegIFN-α plus ribavirin causes graft rejection in over 30% of patients, leading to graft failure and death. Thus, DAA therapy is preferred over interferon-containing therapy in renal transplant patients [371,372]. No data on the situation of transplants of the heart, lung, pancreas, small intestine, or cornea are available. When antiviral therapy is needed, DAA therapy is preferred over interferon-containing therapy. [Recommendations] (Table 12) 1. Antiviral therapy can prevent graft infection in patients awaiting liver transplantation, and should follow the recommendations according to liver function and HCV genotype (B1). 2. All patients who develop recurrent HCV infection after liver transplantation should be prioritized for antiviral therapy (A1). Antiviral treatment should be started as soon as possible when fibrosing cholestatic fibrosis, advanced fibrosis or portal hypertension is noted, since these conditions predict a rapid progression of liver diseases and graft failure (A1). 3. Treatment of patients who develop recurrent HCV infection after liver transplantation: HCV genotype 1 or 4 1) Daily fixed-dose combination ledipasvir (90 mg)/sofosbuvir (400 mg) and weight-based ribavirin (1,200 mg in patients ≥ 75 kg, 1,000 mg in patients 25 IU/mL), it should be retested at week 6 and if it is elevated by greater than 10-fold at week 6, discontinuation of treatment should be considered (C1). 3. To evaluate each individual’s therapeutic response and to modify the duration of treatment during PegIFN-α and ribavirin combination therapy, serum HCV RNA assays should be performed at weeks 4, 12, and 24 of treatment or at the end of treatment, depending on the HCV genotype (B1). In cases of genotype 1, treatment should be stopped in patients who fail to achieve an EVR (A1). Patients who achieve a cEVR can be treated for 48 weeks (A1). Patients with a pEVR should be re-tested at week 24; if HCV RNA remains positive, treatment should be stopped (A1). 4. HCV RNA should be measured at 12 or 24 weeks after the cessation of treatment to evaluate therapeutic effects and to identify the achievement of an SVR (A1). 5. Continuous undetectable HCV RNA after achieving an SVR can be regarded as complete eradication of HCV (C1). 6. Risks of hepatocellular carcinoma and complications of chronic liver disease remain after achieving an SVR in patients with cirrhosis or advanced hepatic fibrosis, and continuous management and surveillance following the strategies for chronic liver disease are needed (B1). 7. If an SVR is not achieved, continuous management of chronic liver disease is necessary (B1). Adverse effects of antiviral therapy and their management A thorough education about the process of treatment, and expected adverse reactions and their management encourages patients to continue treatment. Adverse reactions should be assessed at week 2-4 of treatment, and progress should be monitored at about 4-12-week intervals. DAA has fewer adverse reactions and tends to be well tolerated. Commonly reported adverse reactions are fatigue, headache, nausea, etc.; however, less than 1% of patients stop treatment due to adverse reactions. Adverse reactions of each drug are described in detail in the section ‘New drugs, direct-acting antivirals (DAA)’. A liver function test is performed within 4 weeks of treatment, and stopping of treatment may be considered when ALT is over 10-fold higher than the upper limit of the normal range, or when there is a risk of acute liver failure, such as an elevated bilirubin level or extended prothrombin time, even if the elevation of ALT is insignificant. More than 20% of patients treated with PegIFN-α and ribavirin combination therapy experience headache, fever, myalgia, muscular rigidity, arthralgia, nausea, anorexia, weight loss, diarrhea, hair loss, skin rash, pruritus, inflammation at sites of injection, dyspnea, fatigue, insomnia, irritability, or depression (Table 13) [254,261,262,398]. However, the severity and/or frequency of these adverse effects may vary, as these were reported by patients enrolled in clinical trials [398]. Adverse effects after PegIFN-α injection can be classified as flulike symptoms, myelosuppression, neuropsychological problems, and autoimmune dysfunction. Flu-like symptoms including fever, fatigue, myalgia, or nausea occur in ~37% of patients,254,261,262,398 but these symptoms can be alleviated by administration of analgesics and usually improve 4-6 weeks after treatment [398]. Myelosuppression causes neutropenia and thrombocytopenia, the main causes of dose reduction, and often set the therapeutic limit in patients with cirrhosis. Dose of PegIFN-α should be reduced or skipped in cases of severe adverse effects. Especially, when the absolute neutrophil count decreases to 500/mm3 to avoid drug-drug interactions until HCV treatment is completed. In patients with a CD4 lymphocyte count 18 months since maternal antibodies can be delivered to newborns. If an earlier assay is required, HCV RNA assay may be considered after 6 months of age (B2). 3. HCV infected children aged 3-17 years should be considered appropriate candidates for treatment according to the same criteria used in adults (B1). 4. The dose of PegIFN-α is 60 μg/1.73 m2/week for 2b and 180 μg/1.73 m2/week for 2a, and the dose of ribavirin is 15 mg/kg/day. Genotype 1 and 4 patients should be treated for 48 weeks and genotype 2 and 3 patients should be treated for 24 weeks (B1).