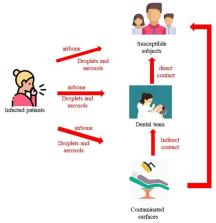
The city of Wuhan in China is the focus of global attention due to an outbreak of a febrile respiratory illness due to a coronavirus 2019-nCoV. In December 2019, there was an outbreak of pneumonia of unknown cause in Wuhan, Hubei province in China, with an epidemiological link to the Huanan Seafood Wholesale Market where there was also sale of live animals. Notification of the WHO on 31 Dec 2019 by the Chinese Health Authorities has prompted health authorities in Hong Kong, Macau, and Taiwan to step up border surveillance, and generated concern and fears that it could mark the emergence of a novel and serious threat to public health (WHO, 2020a, Parr, 2020). The Chinese health authorities have taken prompt public health measures including intensive surveillance, epidemiological investigations, and closure of the market on 1 Jan 2020. SARS-CoV, MERS-CoV, avian influenza, influenza and other common respiratory viruses were ruled out. The Chinese scientists were able to isolate a 2019-nCoV from a patient within a short time on 7 Jan 2020 and perform genome sequencing of the 2019-nCoV. The genetic sequence of the 2019-nCoV has become available to the WHO on 12 Jan 2020 and this has facilitated the laboratories in different countries to produce specific diagnostic PCR tests for detecting the novel infection (WHO, 2020b). The 2019-nCoV is a β CoV of group 2B with at least 70% similarity in genetic sequence to SARS-CoV and has been named 2019-nCoV by the WHO. SARS is a zoonosis caused by SARS-CoV, which first emerged in China in 2002 before spreading to 29 countries/regions in 2003 through a travel-related global outbreak with 8,098 cases with a case fatality rate of 9.6%. Nosocomial transmission of SARS-CoV was common while the primary reservoir was putatively bats, although unproven as the actual source and the intermediary source was civet cats in the wet markets in Guangdong (Hui and Zumla, 2019). MERS is a novel lethal zoonotic disease of humans endemic to the Middle East, caused by MERS-CoV. Humans are thought to acquire MERS-CoV infection though contact with camels or camel products with a case fatality rate close to 35% while nosocomial transmission is also a hallmark (Azhar et al., 2019). The recent outbreak of clusters of viral pneumonia due to a 2019-nCoV in the Wuhan market poses significant threats to international health and may be related to sale of bush meat derived from wild or captive sources at the seafood market. As of 10 Jan 2020, 41 patients have been diagnosed to have infection by the 2019-nCoV animals. The onset of illness of the 41 cases ranges from 8 December 2019 to 2 January 2020. Symptoms include fever (>90% cases), malaise, dry cough (80%), shortness of breath (20%) and respiratory distress (15%). The vital signs were stable in most of the cases while leucopenia and lymphopenia were common. Among the 41 cases, six patients have been discharged, seven patients are in critical care and one died, while the remaining patients are in stable condition. The fatal case involved a 61 year-old man with an abdominal tumour and cirrhosis who was admitted to a hospital due to respiratory failure and severe pneumonia. The diagnoses included severe pneumonia, acute respiratory distress syndrome, septic shock and multi-organ failure. The 2019-nCoV infection in Wuhan appears clinically milder than SARS or MERS overall in terms of severity, case fatality rate and transmissibility, which increases the risk of cases remaining undetected. There is currently no clear evidence of human to human transmission. At present, 739 close contacts including 419 healthcare workers are being quarantined and monitored for any development of symptoms (WHO, 2020b, Center for Health Protection and HKSAR, 2020). No new cases have been detected in Wuhan since 3 January 2020. However the first case outside China was reported on 13th January 2020 in a Chinese tourist in Thailand with no epidemiological linkage to the Huanan Seafood Wholesale Market. The Chinese Health Authorities have carried out very appropriate and prompt response measures including active case finding, and retrospective investigations of the current cluster of patients which have been completed; The Huanan Seafood Wholesale Market has been temporarily closed to carry out investigation, environmental sanitation and disinfection; Public risk communication activities have been carried out to improve public awareness and adoption of self-protection measures. Technical guidance on novel coronavirus has been developed and will continue to be updated as additional information becomes available. However, many questions about the new coronavirus remain. While it appears to be transmitted to humans via animals, the specific animals and other reservoirs need to be identified, the transmission route, the incubation period and characteristics of the susceptible population and survival rates. At present, there is however very limited clinical information of the 2019-nCoV infection and data are missing in regard to the age range, animal source of the virus, incubation period, epidemic curve, viral kinetics, transmission route, pathogenesis, autopsy findings and any treatment response to antivirals among the severe cases. Once there is any clue to the source of animals being responsible for this outbreak, global public health authorities should examine the trading route and source of movement of animals or products taken from the wild or captive conditions from other parts to Wuhan and consider appropriate trading restrictions or other control measures to limit. The rapid identification and containment of a novel coronavirus virus in a short period of time is a re-assuring and a commendable achievement by China’s public health authorities and reflects the increasing global capacity to detect, identify, define and contain new outbreaks. The latest analysis show that the Wuhan CoV cluster with the SARS CoV.10 (Novel coronavirus - China (01): (HU) WHO, phylogenetic tree Archive Number: 20200112.6885385). This outbreak brings back memories of the novel coronavirus outbreak in China, the severe acute respiratory syndrome (SARS) in China in 2003, caused by a novel SARS-CoV-coronavirus (World Health Organization, 2019a). SARS-CoV rapidly spread from southern China in 2003 and infected more than 3000 people, killing 774 by 2004, and then disappeared – never to be seen again. However, The Middle East Respiratory Syndrome (MERS) Coronavirus (MERS-CoV) (World Health Organization, 2019b), a lethal zoonotic pathogen that was first identified in humans in the Kingdom of Saudi Arabia (KSA) in 2012 continues to emerge and re-emerge through intermittent sporadic cases, community clusters and nosocomial outbreaks. Between 2012 and December 2019, a total of 2465 laboratory-confirmed cases of MERS-CoV infection, including 850 deaths (34.4% mortality) were reported from 27 countries to WHO, the majority of which were reported by KSA (2073 cases, 772 deaths. Whilst several important aspects of MERS-CoV epidemiology, virology, mode of transmission, pathogenesis, diagnosis, clinical features, have been defined, there remain many unanswered questions, including source, transmission and epidemic potential. The Wuhan outbreak is a stark reminder of the continuing threat of zoonotic diseases to global health security. More significant and better targeted investments are required for a more concerted and collaborative global effort, learning from experiences from all geographical regions, through a ‘ONE-HUMAN-ENIVRONMENTAL-ANIMAL-HEALTH’ global consortium to reduce the global threat of zoonotic diseases (Zumla et al., 2016). Sharing experience and learning from all geographical regions and across disciplines will be key to sustaining and further developing the progress being made. Author declarations All authors have a specialist interest in emerging and re-emerging pathogens. FN, RK, OD, GI, TDMc, CD and AZ are members of the Pan-African Network on Emerging and Re-emerging Infections (PANDORA-ID-NET) funded by the European and Developing Countries Clinical Trials Partnership the EU Horizon 2020 Framework Programme for Research and Innovation. AZ is a National Institutes of Health Research senior investigator. All authors declare no conflicts of interest.