- Record: found
- Abstract: found
- Article: found
Reversible Photoregulation of Cell–Cell Adhesions With Opto-E-cadherin
Read this article at
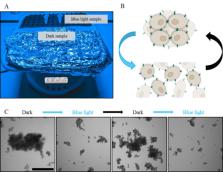
Abstract
The cell–cell adhesion molecule E-cadherin has been intensively studied due to its prevalence in tissue function and its spatiotemporal regulation during epithelial-to-mesenchymal cell transition. Nonetheless, regulating and studying the dynamics of it has proven challenging. We developed a photoswitchable version of E-cadherin, named opto-E-cadherin, which can be toggled OFF with blue light illumination and back ON in the dark. Herein, we describe easy-to-use methods to test and characterise opto-E- cadherin cell clones for downstream experiments.
Key features
• This protocol describes how to implement optogenetic cell–cell adhesion molecules effectively (described here on the basis of opto-E-cadherin), while highlighting possible pitfalls.
• Utilises equipment commonly found in most laboratories with high ease of use.
• Phenotype screening is easy and done within a few hours (comparison of cell clusters in the dark vs. blue light in an aggregation assay).
• Three different functionality assay systems are described.
• After the cell line is established, all experiments can be performed within three days.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Epithelial-mesenchymal transitions in development and disease.
- Record: found
- Abstract: found
- Article: not found
Cell adhesion: the molecular basis of tissue architecture and morphogenesis.
- Record: found
- Abstract: found
- Article: not found