- Record: found
- Abstract: found
- Article: found
Lipid rafts and human diseases: why we need to target gangliosides
Read this article at
Abstract
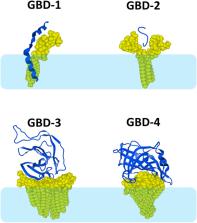
Gangliosides are functional components of membrane lipid rafts that control critical functions in cell communication. Many pathologies involve raft gangliosides, which therefore represent an approach of choice for developing innovative therapeutic strategies. Beginning with a discussion of what a disease is (and is not), this review lists the major human pathologies that involve gangliosides, which includes cancer, diabetes, and infectious and neurodegenerative diseases. In most cases, the problem is due to a protein whose binding to gangliosides either creates a pathological condition or impairs a physiological function. Then, I draw up an inventory of the different molecular mechanisms of protein‐ganglioside interactions. I propose to classify the ganglioside‐binding domains of proteins into four categories, which I name GBD‐1, GBD‐2, GBD‐3, and GBD‐4. This structural and functional classification could help to rationalize the design of innovative molecules capable of disrupting the binding of selected proteins to gangliosides without generating undesirable effects. The biochemical specificities of gangliosides expressed in the human brain must also be taken into account to improve the reliability of animal models (or any animal‐free alternative) of Alzheimer's and Parkinson's diseases.
Abstract
Membrane gangliosides are involved in many diseases, often through a modification of lipid raft homeostasis due to the binding of a pathological protein. Thus, these diseases should be considered as membrane disorders. This article develops the ‘ganglioside‐targeting therapy’ concept for treating such diseases with innovative molecules capable of selectively disrupting the binding of pathological proteins to gangliosides.
Related collections
Most cited references198
- Record: found
- Abstract: found
- Article: not found
Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies.
- Record: found
- Abstract: found
- Article: not found
Structural basis of membrane disruption and cellular toxicity by α-synuclein oligomers
- Record: found
- Abstract: found
- Article: found
