- Record: found
- Abstract: found
- Article: not found
Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization
Read this article at
Abstract
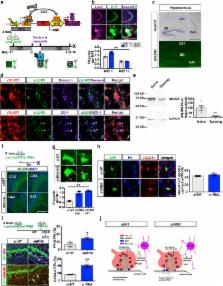
Memories become less precise and generalize over time as memory traces re-organize in hippocampal-cortical networks. Increased time-dependent loss of memory precision characterizes overgeneralization of fear in post-traumatic stress disorder (PTSD) and age-related cognitive impairments. In the hippocampal dentate gyrus (DG), memories are thought to be encoded by so-called “engram-bearing” dentate granule cells (eDGCs). Here we show using rodents that contextual fear conditioning increases connectivity between eDGCs and inhibitory interneurons in the downstream hippocampal CA3 region. We identify actin-binding LIM protein 3 (abLIM3) as a mossy fiber terminal localized cytoskeletal factor, whose levels decrease upon learning. Downregulation of abLIM3 in DGCs was sufficient to increase connectivity with CA3 stratum lucidum interneurons (SLINs), promote parvalbumin (PV) SLIN activation, enhance feed-forward inhibition onto CA3, and maintain a fear memory engram in the dentate gyrus (DG) over time. Furthermore, abLIM3 downregulation in DGCs conferred conditioned context-specific reactivation of memory traces in hippocampal-cortical and amygdalar networks and decreased fear memory generalization at remote time points. Consistent with age-related hyperactivity of CA3, learning failed to increase DGC-SLIN connectivity in 17 month-old mice, whereas abLIM3 downregulation was sufficient to restore DGC-SLIN connectivity, increase PV-SLIN activation and improve remote memory precision. These studies exemplify a connectivity-based strategy targeting a molecular brake of feedforward inhibition in DG-CA3 that may be harnessed to decrease time-dependent memory generalization in PTSD and improve memory precision in aging.
Related collections
Most cited references55
- Record: found
- Abstract: found
- Article: not found
Optogenetic stimulation of a hippocampal engram activates fear memory recall
- Record: found
- Abstract: found
- Article: not found
Awake hippocampal sharp-wave ripples support spatial memory.
- Record: found
- Abstract: found
- Article: not found
