- Record: found
- Abstract: found
- Article: found
Silent disease progression in clinically stable heart failure
Read this article at
Abstract
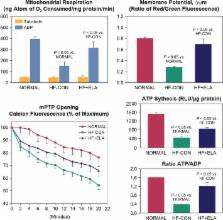
Heart failure with reduced ejection fraction ( HFrEF) is a progressive disorder whereby cardiac structure and function continue to deteriorate, often despite the absence of clinically apparent signs and symptoms of a worsening disease state. This silent yet progressive nature of HFrEF can contribute to the increased risk of death—even in patients who are ‘clinically stable’, or who are asymptomatic or only mildly symptomatic—because it often goes undetected and/or undertreated. Current therapies are aimed at improving clinical symptoms, and several agents more directly target the underlying causes of disease; however, new therapies are needed that can more fully address factors responsible for underlying progressive cardiac dysfunction. In this review, mechanisms that drive HFrEF, including ongoing cardiomyocyte loss, mitochondrial abnormalities, impaired calcium cycling, elevated LV wall stress, reactive interstitial fibrosis, and cardiomyocyte hypertrophy, are discussed. Additionally, limitations of current HF therapies are reviewed, with a focus on how these therapies are designed to counteract the deleterious effects of compensatory neurohumoral activation but do not fully prevent disease progression. Finally, new investigational therapies that may improve the underlying molecular, cellular, and structural abnormalities associated with HF progression are reviewed.
Related collections
Most cited references82
- Record: found
- Abstract: found
- Article: not found
Effect of nesiritide in patients with acute decompensated heart failure.
- Record: found
- Abstract: found
- Article: not found
Aging-associated cardiovascular changes and their relationship to heart failure.
- Record: found
- Abstract: found
- Article: not found