- Record: found
- Abstract: found
- Article: found
Microbial Utilization of Next-Generation Feedstocks for the Biomanufacturing of Value-Added Chemicals and Food Ingredients
Read this article at
Abstract
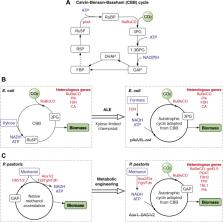
Global shift to sustainability has driven the exploration of alternative feedstocks beyond sugars for biomanufacturing. Recently, C1 (CO 2, CO, methane, formate and methanol) and C2 (acetate and ethanol) substrates are drawing great attention due to their natural abundance and low production cost. The advances in metabolic engineering, synthetic biology and industrial process design have greatly enhanced the efficiency that microbes use these next-generation feedstocks. The metabolic pathways to use C1 and C2 feedstocks have been introduced or enhanced into industrial workhorses, such as Escherichia coli and yeasts, by genetic rewiring and laboratory evolution strategies. Furthermore, microbes are engineered to convert these low-cost feedstocks to various high-value products, ranging from food ingredients to chemicals. This review highlights the recent development in metabolic engineering, the challenges in strain engineering and bioprocess design, and the perspectives of microbial utilization of C1 and C2 feedstocks for the biomanufacturing of value-added products.
Related collections
Most cited references133
- Record: found
- Abstract: not found
- Article: not found
Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. technological use of CO2.
- Record: found
- Abstract: found
- Article: not found
Clostridium ljungdahlii represents a microbial production platform based on syngas.
- Record: found
- Abstract: found
- Article: not found