- Record: found
- Abstract: found
- Article: found
Ketamine—50 years in use: from anesthesia to rapid antidepressant effects and neurobiological mechanisms
Read this article at
Abstract
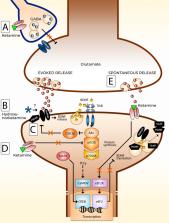
Over the past 50 years, ketamine has solidified its position in both human and veterinary medicine as an important anesthetic with many uses. More recently, ketamine has been studied and used for several new indications, ranging from chronic pain to drug addiction and post-traumatic stress disorder. The discovery of the rapid-acting antidepressant effects of ketamine has resulted in a surge of interest towards understanding the precise mechanisms driving its effects. Indeed, ketamine may have had the largest impact for advancements in the research and treatment of psychiatric disorders in the past few decades. While intense research efforts have been aimed towards uncovering the molecular targets underlying ketamine’s effects in treating depression, the underlying neurobiological mechanisms remain elusive. These efforts are made more difficult by ketamine’s complex dose-dependent effects on molecular mechanisms, multiple pharmacologically active metabolites, and a mechanism of action associated with the facilitation of synaptic plasticity. This review aims to provide a brief overview of the different uses of ketamine, with an emphasis on examining ketamine’s rapid antidepressant effects spanning molecular, cellular, and network levels. Another focus of the review is to offer a perspective on studies related to the different doses of ketamine used in antidepressant research. Finally, the review discusses some of the latest hypotheses concerning ketamine’s action.
Related collections
Most cited references248
- Record: found
- Abstract: found
- Article: not found
NMDA Receptor Blockade at Rest Triggers Rapid Behavioural Antidepressant Responses
- Record: found
- Abstract: found
- Article: not found
NMDAR inhibition-independent antidepressant actions of ketamine metabolites
- Record: found
- Abstract: found
- Article: not found