- Record: found
- Abstract: found
- Article: found
Ketamine administration ameliorates anesthesia and surgery-induced cognitive dysfunction via activation of TRPV4 channel opening
Read this article at
Abstract
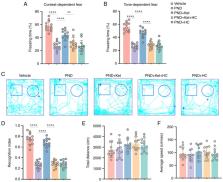
Perioperative neurocognitive disorder (PND) is a common complication associated with anesthesia and surgery in the elderly. The dysfunction of transient receptor potential vanilloid 4 (TRPV4) has been associated with a number of diseases, including Alzheimer's disease. Given that ketamine can reportedly improve PNDs, the present study sought to determine whether ketamine-induced PND alleviation was mediated by activation of TRPV4 channel opening. A total of 120, 20-month-old male C57BL/6 mice were randomly divided into five groups: Vehicle, PND (tibial fracture surgery), PND + ketamine (Ket), PND + Ket + HC-067047 (HC), and PND + HC groups. Ketamine (0.5 mg/kg) was administered intraperitoneally once a day for 3 days after surgery and HC-067047 (1 µmol/2 µl), an antagonist of TRPV4, was administered via the left lateral ventricle 30 min before ketamine treatment. Superoxide dismutase (SOD), malondialdehyde (MDA), lipid peroxidation (LPO), IL-1β, IL-6, adenosine monophosphate-activated protein kinase (AMPK), NF-κB, TNF-α and IFN-β levels were determined 3 days after surgery. At 28 days after surgery, fear conditioning and novel object recognition were assessed, and Aβ1-42 levels were measured and ionized calcium binding adaptor molecule 1 (Iba1) staining was conducted on day 31 after surgery. The results revealed that ketamine administration upregulated total SOD activity, downregulated MDA and LPO content, mitigated phosphorylated (p)-NF-κB, TNF-α mRNA and IFN-β mRNA expression in the hippocampus, and promoted p-AMPK 3 days after surgery. Furthermore, it was found that ketamine increased both context- and tone-dependent fear conditioning, and the time spent exploring a novel object, and reduced Aβ peptide levels and microglial activation 30 days after surgery. Notably, these changes could be reversed by HC-067047 to a certain extent. In conclusion, ketamine improved PND in aged mice after tibial fracture surgery and the potential mechanism may involve activation of the TRPV4/AMPK/NF-κB signaling pathway.
Related collections
Most cited references79
- Record: found
- Abstract: found
- Article: not found
Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method.
- Record: found
- Abstract: not found
- Article: not found
Animal research: reporting in vivo experiments: the ARRIVE guidelines.
- Record: found
- Abstract: found
- Article: not found