- Record: found
- Abstract: found
- Article: found
Direct Binding of Retromer to Human Papillomavirus Type 16 Minor Capsid Protein L2 Mediates Endosome Exit during Viral Infection
Read this article at
Abstract
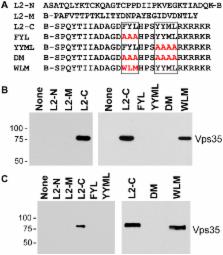
Trafficking of human papillomaviruses to the Golgi apparatus during virus entry requires retromer, an endosomal coat protein complex that mediates the vesicular transport of cellular transmembrane proteins from the endosome to the Golgi apparatus or the plasma membrane. Here we show that the HPV16 L2 minor capsid protein is a retromer cargo, even though L2 is not a transmembrane protein. We show that direct binding of retromer to a conserved sequence in the carboxy-terminus of L2 is required for exit of L2 from the early endosome and delivery to the trans-Golgi network during virus entry. This binding site is different from known retromer binding motifs and can be replaced by a sorting signal from a cellular retromer cargo. Thus, HPV16 is an unconventional particulate retromer cargo, and retromer binding initiates retrograde transport of viral components from the endosome to the trans-Golgi network during virus entry. We propose that the carboxy-terminal segment of L2 protein protrudes through the endosomal membrane and is accessed by retromer in the cytoplasm.
Author Summary
The human papillomaviruses are important carcinogens, but little is known about how these non-enveloped viruses traffic to the nucleus, the site of genome replication. We use imaging, biochemical, and genetic techniques to show that a multi-subunit intracellular trafficking machine known as retromer binds directly to the L2 minor capsid protein in the virus particle to initiate its transport from the endosome to other membrane-bound organelles farther inside the cell. Most notably, knock-down of retromer expression or mutation of newly identified retromer binding sites in L2 cause the accumulation of incoming HPV16 capsids in the endosome and prevent trafficking to the Golgi. These defects can be corrected by insertion of a retromer binding site from a cellular cargo. Because all previously known retromer cargoes are cellular transmembrane proteins, the virus represents a new class of retromer cargo. In addition to elucidating the mechanism of viral endosome escape, these results suggest that retromer may play a more versatile role in cell biology than previously recognized.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Chapter 2: The burden of HPV-related cancers.
- Record: found
- Abstract: found
- Article: not found
Human papillomavirus infection requires cell surface heparan sulfate.
- Record: found
- Abstract: found
- Article: not found