- Record: found
- Abstract: not found
- Article: not found
Enzyme-Instructed Intracellular Peptide Assemblies
October 26 2023
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Related collections
Most cited references67
- Record: found
- Abstract: found
- Article: found
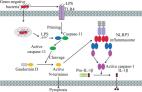
Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors
Yang Yang, Huanan Wang, Mohammed Kouadir … (2019)

- Record: found
- Abstract: found
- Article: found
DNA-induced liquid phase condensation of cGAS activates innate immune signaling
Mingjian Du, Zhijian Chen (2018)