- Record: found
- Abstract: found
- Article: found
Adverse Maternal Environment and Postweaning Western Diet Alter Hepatic CD36 Expression and Methylation Concurrently with Nonalcoholic Fatty Liver Disease in Mouse Offspring
Read this article at
ABSTRACT
Background
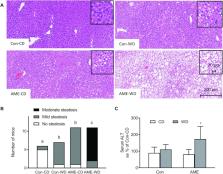
The role of an adverse maternal environment (AME) in conjunction with a postweaning Western diet (WD) in the development of nonalcoholic fatty liver disease (NAFLD) in adult offspring has not been explored. Likewise, the molecular mechanisms associated with AME-induced NAFLD have not been studied. The fatty acid translocase or cluster of differentiation 36 (CD36) has been implicated to play a causal role in the pathogenesis of WD-induced steatosis. However, it is unknown if CD36 plays a role in AME-induced NAFLD.
Objective
This study was designed to evaluate the isolated and additive impact of AME and postweaning WD on the expression and DNA methylation of hepatic Cd36 in association with the development of NAFLD in a novel mouse model.
Methods
AME constituted maternal WD and maternal stress, whereas the control (Con) group had neither. Female C57BL/6J mice were fed a WD [40% fat energy, 29.1% sucrose energy, and 0.15% cholesterol (wt/wt)] 5 wk prior to pregnancy and throughout lactation. Non invasive variable stressors (random frequent cage changing, limited bedding, novel object, etc.) were applied to WD dams during the last third of pregnancy to produce an AME. Con dams consumed the control diet (CD) (10% fat energy, no sucrose or cholesterol) and were not exposed to stress. Male offspring were weaned onto either CD or WD, creating 4 experimental groups: Con-CD, Con-WD, AME-CD, and AME-WD, and evaluated for metabolic and molecular parameters at 120 d of age.
Related collections
Most cited references65
- Record: found
- Abstract: found
- Article: not found
Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man.
- Record: found
- Abstract: found
- Article: not found
Design and validation of a histological scoring system for nonalcoholic fatty liver disease.
- Record: found
- Abstract: not found
- Article: not found