- Record: found
- Abstract: found
- Article: found
Nanotherapy delivery of c-myc inhibitor targets Protumor Macrophages and preserves Antitumor Macrophages in Breast Cancer
Read this article at
Abstract
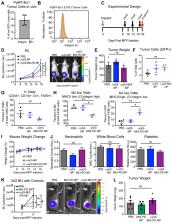
Tumor-associated macrophages (TAMs) enhance tumor growth in mice and are correlated with a worse prognosis for breast cancer patients. While early therapies sought to deplete all macrophages, current therapeutics aim to reprogram pro-tumor macrophages (M2) and preserve those necessary for anti-tumor immune responses (M1). Recent studies have shown that c-MYC (MYC) is induced in M2 macrophages in vitro and in vivo where it regulates the expression of tumor-promoting genes. In a myeloid lineage MYC KO mouse model, MYC had important roles in macrophage maturation and function leading to reduced tumor growth. We therefore hypothesized that targeted delivery of a MYC inhibitor to established M2 TAMs could reduce polarization toward an M2 phenotype in breast cancer models.
Methods: In this study, we developed a MYC inhibitor prodrug (MI3-PD) for encapsulation within perfluorocarbon nanoparticles, which can deliver drugs directly to the cytosol of the target cell through a phagocytosis independent mechanism. We have previously shown that M2-like TAMs express significant levels of the vitronectin receptor, integrin β3, and in vivo targeting and therapeutic potential was evaluated using αvβ3 integrin targeted rhodamine-labeled nanoparticles (NP) or integrin αvβ3-MI3-PD nanoparticles.
Results: We observed that rhodamine, delivered by αvβ3-rhodamine NP, was incorporated into M2 tumor promoting macrophages through both phagocytosis-independent and dependent mechanisms, while NP uptake in tumor suppressing M1 macrophages was almost exclusively through phagocytosis. In a mouse model of breast cancer (4T1-GFP-FL), M2-like TAMs were significantly reduced with αvβ3-MI3-PD NP treatment. To validate this effect was independent of drug delivery to tumor cells and was specific to the MYC inhibitor, mice with integrin β3 knock out tumors (PyMT-Bo1 β3KO) were treated with αvβ3-NP or αvβ3-MI3-PD NP. M2 macrophages were significantly reduced with αvβ3-MI3-PD nanoparticle therapy but not αvβ3-NP treatment.
Conclusion: These data suggest αvβ3-NP-mediated drug delivery of a c-MYC inhibitor can reduce protumor M2-like macrophages while preserving antitumor M1-like macrophages in breast cancer.
Related collections
Most cited references62
- Record: found
- Abstract: found
- Article: not found
The Gene Expression Omnibus Database.
- Record: found
- Abstract: found
- Article: not found
Transcription factors as targets for cancer therapy.
- Record: found
- Abstract: found
- Article: not found