- Record: found
- Abstract: found
- Article: found
Dysregulation of Mitochondrial Ca 2+ Uptake and Sarcolemma Repair Underlie Muscle Weakness and Wasting in Patients and Mice Lacking MICU1
Read this article at
SUMMARY
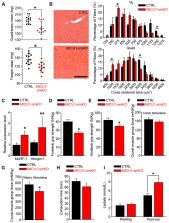
Muscle function is regulated by Ca 2+, which mediates excitation-contraction coupling, energy metabolism, adaptation to exercise, and sarcolemmal repair. Several of these actions rely on Ca 2+ delivery to the mitochondrial matrix via the mitochondrial Ca 2+ uniporter, the pore of which is formed by mitochondrial calcium uniporter (MCU). MCU’s gatekeeping and cooperative activation are controlled by MICU1. Loss-of-protein mutation in MICU1 causes a neuromuscular disease. To determine the mechanisms underlying the muscle impairments, we used MICU1 patient cells and skeletal muscle-specific MICU1 knockout mice. Both these models show a lower threshold for MCU-mediated Ca 2+ uptake. Lack of MICU1 is associated with impaired mitochondrial Ca 2+ uptake during excitation-contraction, aerobic metabolism impairment, muscle weakness, fatigue, and myofiber damage during physical activity. MICU1 deficit compromises mitochondrial Ca 2+ uptake during sarcolemmal injury, which causes ineffective repair of the damaged myofibers. Thus, dysregulation of mitochondrial Ca 2+ uptake hampers myofiber contractile function, likely through energy metabolism and membrane repair.
Graphical Abstract
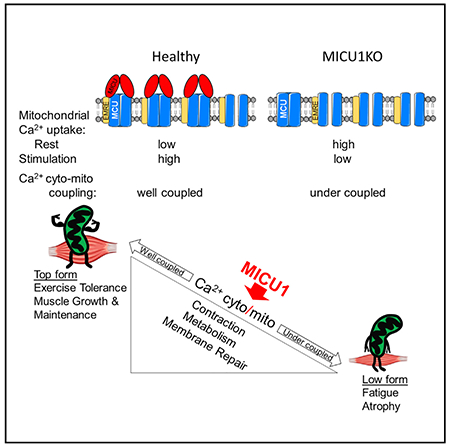
In Brief
Debattisti et al. report that skeletal muscle-specific loss of mitochondrial Ca 2+ uptake 1 (MICU1) in mouse impairs mitochondrial calcium signaling, energy metabolism, and membrane repair, leading to muscle weakness, fatigue, myofiber damage, and high CK levels, recapitulating the muscle symptoms of MICU1 loss in patients.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake
- Record: found
- Abstract: found
- Article: not found
EMRE is an essential component of the mitochondrial calcium uniporter complex.
- Record: found
- Abstract: found
- Article: not found