- Record: found
- Abstract: found
- Article: found
The Mitochondrial Ca 2+ Uptake and the Fine-Tuning of Aerobic Metabolism
Read this article at
Abstract
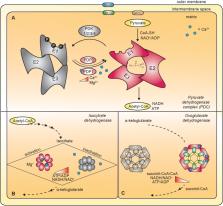
Recently, the role of mitochondrial activity in high-energy demand organs and in the orchestration of whole-body metabolism has received renewed attention. In mitochondria, pyruvate oxidation, ensured by efficient mitochondrial pyruvate entry and matrix dehydrogenases activity, generates acetyl CoA that enters the TCA cycle. TCA cycle activity, in turn, provides reducing equivalents and electrons that feed the electron transport chain eventually producing ATP. Mitochondrial Ca 2+ uptake plays an essential role in the control of aerobic metabolism. Mitochondrial Ca 2+ accumulation stimulates aerobic metabolism by inducing the activity of three TCA cycle dehydrogenases. In detail, matrix Ca 2+ indirectly modulates pyruvate dehydrogenase via pyruvate dehydrogenase phosphatase 1, and directly activates isocitrate and α-ketoglutarate dehydrogenases. Here, we will discuss the contribution of mitochondrial Ca 2+ uptake to the metabolic homeostasis of organs involved in systemic metabolism, including liver, skeletal muscle, and adipose tissue. We will also tackle the role of mitochondrial Ca 2+ uptake in the heart, a high-energy consuming organ whose function strictly depends on appropriate Ca 2+ signaling.
Related collections
Most cited references116
- Record: found
- Abstract: found
- Article: not found
Inflammation and metabolic disorders.
- Record: found
- Abstract: found
- Article: not found
Directed evolution of APEX2 for electron microscopy and proteomics
- Record: found
- Abstract: found
- Article: not found