- Record: found
- Abstract: found
- Article: found
Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis
Read this article at
Abstract
Background
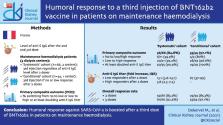
Humoral response against sudden acute respiratory syndrome coronavirus 2 (SARS-CoV-2) after two doses of BNT162b2 (Pfizer-BioNTech) has been proven to be less intense in maintenance dialysis patients as compared with healthy subjects, leading the French authorities to recommend a third injection in this population. Here we investigated the response to the third injection in two cohorts of haemodialysis (HD) patients.
Methods
Data from two prospective observational cohorts were collected. In the first (‘systematic’) cohort, patients from two HD centres ( n = 66) received a third injection of BNT162b2, regardless of the response after two injections. In the second (‘conditional’) cohort, the injection was only prescribed to patients ( n = 34) with no or low response to the previous two doses. In both cohorts, the third dose was injected 1–2 months after the second dose. Serology was performed after the second and third doses to assess anti-Spike immunoglobulin G (S IgG) antibody titre.
Results
In the systematic cohort, anti-S IgG was found in 83.3 and 92.4% of patients after the second and third doses of BNT162b2, respectively. In this cohort, 6/11 (54.5%) and 20/21 (95.2%) patients switched from non-responder to low responder and from low responder to high responder, respectively. In low and high responders to two doses, 50/55 (90.9%) at least doubled their anti-S IgG titre. Similar trends were observed in the conditional cohort.
Graphical Abstract
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe.
- Record: found
- Abstract: found
- Article: not found
Humoral Response to the Pfizer BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis
- Record: found
- Abstract: not found
- Article: not found
