- Record: found
- Abstract: found
- Article: found
Echocardiographically defined haemodynamic categorization predicts prognosis in ambulatory heart failure patients treated with sacubitril/valsartan
Read this article at
Abstract
Aim
Echo‐derived haemodynamic classification, based on forward‐flow and left ventricular (LV) filling pressure (LVFP) correlates, has been proposed to phenotype patients with heart failure and reduced ejection fraction (HFrEF). To assess the prognostic relevance of baseline echocardiographically defined haemodynamic profile in ambulatory HFrEF patients before starting sacubitril/valsartan.
Methods and results
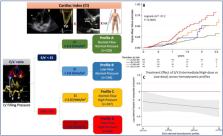
In our multicentre, open‐label study, HFrEF outpatients were classified into 4 groups according to the combination of forward flow (cardiac index; CI:< or ≥2.0 L/min/m 2) and early transmitral Doppler velocity/early diastolic annular velocity ratio (E/e′: ≥ or <15): Profile‐A: normal‐flow, normal‐pressure; Profile‐B: low‐flow, normal‐pressure; Profile‐C: normal‐flow, high‐pressure; Profile‐D: low‐flow, high‐pressure. Patients were started on sacubitril/valsartan and followed‐up for 12.3 months (median). Rates of the composite of death/HF‐hospitalization were assessed by multivariable Cox proportional‐hazards models. Twelve sites enrolled 727 patients (64 ± 12 year old; LVEF: 29.8 ± 6.2%). Profile‐D had more comorbidities and worse renal and LV function. Target dose of sacubitril/valsartan (97/103 mg BID) was more likely reached in Profile‐A (34%) than other profiles (B: 32%, C: 24%, D: 28%, P < 0.001). Event‐rate (per 100 patients per year) progressively increased from Profile‐A to Profile‐D (12.0%, 16.4%, 22.9%, and 35.2%, respectively, P < 0.0001). By covariate‐adjusted Cox model, profiles with low forward‐flow (B and D) remained associated with poor outcome ( P < 0.01). Adding this categorization to MAGGIC‐score and natriuretic peptides, provided significant continuous net reclassification improvement (0.329; P < 0.001). Intermediate and high‐dose sacubitril/valsartan reduced the event's risk independently of haemodynamic profile.
Related collections
Most cited references20
- Record: found
- Abstract: not found
- Article: not found
2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC.
- Record: found
- Abstract: found
- Article: not found
Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging.
- Record: found
- Abstract: found
- Article: not found